A Proposal for Working Group 19 – Dermatologic
Standards
DICOM Whitepaper
Brian C. Madden, Ph.D.
Department of Dermatology
14 December 2011
Abstract
Dermatology is a specialty dominated by visual assessment with the bulk
of care provided through face-to-face examinations. Except for their use in teledermatology
and dermatopathology, images of the skin are not normally involved in
diagnostic decisions. Even so, skin images are being captured in the clinical
setting and many of them end up in the patient record. Their inclusion is problematic
for a number of reasons, not the least of which is the use by dermatologists of
consumer-grade capture and display devices – an unregulated activity that has
the potential to disadvantage the diagnostic decision process. The
implementation of standards for dermatologic imaging would contribute to
improvements in both diagnosis and patient safety.
While the development of new, less capital-equipment-intensive, care
delivery models is at the core of most healthcare savings initiatives, ways
must be found at the same time to maintain the integrity of the care delivery
system through device characterization and process validation for all clinical
specialties. What is required here to support dermatology is research into a complete
diagnostic imaging chain. Data are needed to define the capture, display,
observation, and decision transfer functions. There is a need to account for the
effects consumer devices have on the imaging chain and also to enable the emerging
imaging modalities that have the potential to enhance dermatologic diagnosis.
A solution is needed that will help promote
the digitization of dermatology, as well as the use of consumer off-the-shelf (COTS)
imaging and display devices in several of the other specialties which also
don’t require large capital investments to deliver care. Support may be forthcoming
from the new influences in healthcare delivery – PHRs, EHRs, PACS and
enterprise-wide information and management systems. The synergy here occurs in
the dependency of these healthcare systems on models of integrated medical
information collection and delivery. It is in the interest of the new forces in
healthcare to contribute to the redress of existing economic and technical deficiencies
and thereby to bring imaging in under-supported specialties such as dermatology
all the benefits of digital medicine. This is an effort that would be significantly
advanced by a DICOM dermatologic imaging standard.
Introduction - A little over a decade ago an initial attempt was made to create a DICOM Working Group that would address the needs of dermatologic imaging. That attempt to create WG-19 was ahead of its time. Although the adoption of digital cameras in dermatology was growing in the late 1990s, and growing rapidly, it was not possible to engage the relevant vendors, professional societies, and other interest groups in the effort. A lack of economic, scientific and medical incentives resulted in dermatologists not incorporating computer-based and image-based diagnostic utilities in their practices to the same degree as was observed in some other specialties.
Times have changed. The Patient Protection and Affordable Care Act is now federal law. American medicine is undergoing a long overdue technological tune-up. More than a decade after the first try to create a dermatologic imaging standard, digital imaging in medicine is flourishing. PACS vendors, and more recently EHR and PHR providers, have emerged as a new influence in healthcare with an economic interest in the quality of medical image representation. While the economic impact of dermatology itself is miniscule in the medical equipment marketplace, the need to provide a comprehensive electronic medical record package for healthcare enterprises that covers all specialties is increasingly a significant unifying factor driving clinical IT. [1-3] In addition, the creation of a DICOM dermatologic imaging standard will be the key to enabling efficiencies, functionalities, and improvements in the distribution of scarce healthcare resources not previously possible for this specialty.
Following this introduction, there is a short review of both the advantages and concerns associated with imaging in dermatology (Skin Imaging and Dermatologic Diagnosis), an overview of those applications where digital imaging is being used in dermatologic diagnosis (Dermatopathology, Teledermatology) and where it is not quite yet being used (Modalities Waiting in the Wings). Following these sections is a discussion of the technical imaging issues (Characterization and Validation), a look at what support and Working Group structure might be required for the development of a standard (Business Model, Working Group 19), and ending with a discussion and summary (Conclusions).
Skin Imaging and Dermatologic Diagnosis - Unlike in specialties such as radiology and cardiology which use ionizing radiation to visualize structures within the body or in ophthalmology which images structures in the eye through the cornea and lens using visible light, the surface of the skin is available for direct inspection. Dermatology is among the most visually-based of the medical specialties, yet it has developed a dependence on detailed verbal descriptions. Much of what marks the training of dermatology residents even today is the assimilation of a comprehensive lexicon [4] that describes the spatial luminance and chromatic patterns of the hundreds and hundreds of distinct named skin diseases in all the variations due to anatomic location, sex, and skin color (viz. [5]). The cumbersome capture, development, storage and distribution process of images on film has historically prevented that physical medium from being the conduit of dermatologic diagnostic evidence and, in consequence, has been a major motivation in the continued use of verbal descriptions in the patient record. Given their dependence on physical exam-based dermatologic diagnoses and on clinical observations being recorded in words, dermatologists have been inoculated against the digital imaging revolution to a much larger degree than clinicians in most other specialties.
Initially, when images of the skin were acquired using photographic film, it was done not for diagnostic purposes but rather to create collections of rare, curious or exemplary cases that would help expose dermatology residents to a wide variety of the manifestations of each disease. Expectations have changed with the emergence of high quality digital imaging technologies that have become both economical and accessible. The transition to the use of digital photography in dermatologic diagnosis, either explicitly (teledermatology) or implicitly (the viewing images of skin attached to an EHR for any purpose), will depend on the ability of these images to evoke and to adapt the clinical visual skills learned in conducting physical exams. The strengths and weaknesses of making diagnostic decisions based on images are still being worked out for dermatology.
What is wrong with this picture? As indicated above, although digital images are increasingly being appended to the patient record, the evidence obtained from a face-to-face examination has remained the dominant diagnostic venue. The role of images in the patient record has primarily been one of documentation. Nonetheless, problems may arise from the inclusion of digital images of the skin in EHRs even though they are not intended as a primary source of diagnostic evidence. The entry of images for one purpose may well accrue other uses when the patient record is viewed at a later time. The potential for the co-option of images will only increase as telehealth moves patient data collection away from the hospital and clinic and into the patient’s home. Can images acquired for one purpose (affect, gait) be appropriately used (consciously or not) for assessment in another domain (rash, jaundice)? How is an increase in diagnostic mistakes to be avoided given the availability of images from undocumented devices obtained under uncontrolled conditions? How is the quality of patient care to be maintained without onerously compartmentalizing imaging data?
The relation among diagnostic decisions, camera parameters, and image appearance measures are part of the program of dermatologic image research validation that remains to be developed. An important part of the diagnostic imaging chain that needs to be defined is to reconcile device characterization with the image features of the disease processes that are diagnostic for each condition. The ability to assess the ability of an image to resolve a given diagnostic question would also be greatly enhanced by the incorporation of capture and display process information into the image metadata. The appended record could contain information such as internal settings and external capture conditions or the results of image quality metrics. The creation of an Imaging Utility Graphical User Interface (IUGUI) that could take the images and metadata and combine them with a perceptually-based visual discrimination metric to provide diagnostic and visualization support will provide the basis for much of the standards development and validation. A dermatology IUGUI can provide measurements that assess and utilities that correct inherent flaws in the design of capture and display devices as well as for procedural errors in their handling and configuration. Some of the known relations and parameters are discussed here and in the sections that follow.
To what extent should the use of COTS cameras and displays be a concern for dermatology? The digital point-and-shoot cameras that are so popular with the residents because they easily fit into the pocket of a lab coat can be, in fact, a clinical image quality mine field. Dermatology, as well as several other specialties, has made a risky compromise in the use of inexpensive consumer-grade cameras to capture diagnostic images.[1] In trade for having access to a source of cheap cameras and displays, dermatology has accepted a significantly reduced ability to control the manipulation of the images the cameras and displays produce.
The design of these consumer cameras is driven by cost and aesthetic factors that are very different from, and are often inimical to, diagnostic factors. To minimize costs, the consumer market has moved to put more sensors on a die while putting more pixels on a sensor. Photosites have shrunk from 20 microns to 2 with a corresponding loss of well capacity and this has led to a corresponding reduction in the ability of photosites to store and transfer the pools of electrons that represent pixel amplitudes. In addition in many cases, manufacturers have moved from a camera-specific sensor technology that yielded good signal to noise ratios (CCD) [6] to one that is not so good but is cheaper to fabricate (CMOS). [7-8] To maintain the aesthetic appeal of these images, the cameras now universally incorporate image processing chips [7-8] that can remove noise, enhance edges and boost chromatic saturation. More importantly, not all of these manipulations can be turned off, even when RAW image format is used. [9] Compounding these difficulties, the manufacturers, one and all, treat the processing details as trade secrets and do not reveal the nature or extent of their manipulations.
As an alternative, machine vision cameras are available with performance superior to the consumer devices and, in general, the details of any image manipulations are contained in their published specifications. [10] The higher quality components and the smaller production runs, however, render the cost of these devices prohibitive for dermatology given the current clinical reimbursement schedules.
The use of consumer displays in the diagnostic imaging chain incurs similar trades as those made for consumer cameras. Price determines quality in that the cost of components affects performance. For example, more of the internal transformations can be adjusted in expensive medical-grade displays and there is usually less output variability across the screens of these high-end products than is often found in consumer-grade units. These nonuniformities affect diagnostic performance. While the economies of scale in the consumer market tend to lower price, the pressures of competition also tend to force compromises in device design that while they don’t degrade performance on low demand consumer tasks such as displaying websites already streamlined for fast downloading, their performance adequacy on more demanding tasks such as image-based diagnosis is far less certain.
The trend in consumer cameras and displays is not only moving toward less expensive devices but also to greater mobility. The utility and convenience that come with the increased portability of devices that contain small displays also carry with them an often unrecognized assault on image quality. Setting aside the consequences of the additional image processing that accompanies the conversion of large images to fit on small screens and neglecting the perceptual masking that often accompanies the glare present in uncontrolled clinical viewing environments, the display of as few as 1 in 70 captured pixels when reviewing an image on a camera’s LCD should give pause. In many tasks these small display devices succumb to an aperture effect. There are tasks where the inability to simultaneously appreciate a large proportion of an image leads to a performance deficit for which no amount of scrolling or panning can compensate. Downsizing images to fit on the smaller screens causes loss of diagnostic information due to the prefiltering required to reduce aliasing. It is sobering to consider that many of the current camera LCDs that manifest difficulties in portraying pertinent detail of full-sized captured images have more pixels than the iPhone 4 display, others have more than the iPad.
The specific concern is that there are ill-considered trades that are being made for cost savings in consumer devices and that these compromises may affect diagnostic performance. An example of a clinical concern with consumer-grade imaging devices can be seen in the visibility of any of the black (or blue, brown or gray) dots that are diagnostic for superficial spreading melanoma. These diagnostic details may well be altered to a significant degree because they fall within the bandpass of a potent CMOS noise reduction filter. Another factor that can degrade the visibility of diagnostic detail can come from simple handling. Although many mobile screens are now oleophobic, the degree to which the glass surfaces can disperse the range of body fluids and medications they are likely to encounter in the exam room has yet to be demonstrated. The balance between convenience and the risk of nosocomial infection in the use of these consumer devices is another trade that remains to be negotiated.
The danger of all these degradations is the increased potential for false negatives – confusions that occur because these devices are designed more toward providing cosmetically appealing caricatures than radiometrically accurate reconstructions. The trend over time of these devices to capture diagnostic detail does not appear to be in a good direction. The market influences controlling the design of cameras and displays will only increase the divergence over time in the accuracy of the skin representations as consumer electronics inevitably come under greater and greater pricing constraints.
Much can be learned about avoiding these device pitfalls and about developing good capture and display techniques from the digital advances being made by two auxiliary domains in dermatology: dermatopathology and teledermatology.
Dermatopathology - As a backup to the physical exam, dermatopathology, principally through histology, acts as the gold standard for dermatologic diagnosis. Analogous to the digital transition of dermatology away from film, dermatopathology is moving away from the need to directly view histology slides. Collaboration with Working Group 26 (Pathology) will allow Dermatologic Standards to take advantage of their progress in device characterization and calibration and in image file management. Digital images of skin histology share many of the diagnostic requirements and problems with dermatologic skin imaging for both capture and display. The two approaches use different imaging configurations but they both seek to capture the same sorts of diagnostic detail in tissue patterns at different scales in visible light color images.
The digitization of a section of tissue that can be centimeters wide while maintaining a planarity equal to a tissue thickness of a few micrometers can be a daunting task. Methods that are successful here in the detection and correction of defocus blur can be applied to the defocus caused by handheld capture of a lesion, and vice versa. Similarly, in order to visualize diagnostic detail in digital pathology across the various stains, WG-26 has had to determine what color management methods are required to maintain diagnostic performance. For example, what rendering intents are called for in gamut mapping across different devices? Histology has had to face the same trade in gamut mapping selection as dermatology. Should a mapping be chosen that minimizes algorithmic error by restricting color errors to outliers or one that minimizes perceptual distortion but that increases algorithmic error? Again, reciprocity of interest should hold between this task and the need to extract diagnostic detail from color skin images. In another application, the development of color calibration slides for use in a closed capture environment such as a microscope should closely track the requirements to develop a similar calibration artifact for digital dermoscopy.
While the content of a single skin image captured with a consumer-grade camera can’t compare to the massive file size associated with the digitization of a histology slide, the lessons learned in the effect compression levels have on diagnostic detail in these representations should provide useful guidance for dermatology. Even though the cameras used to digitize pathology are of a much higher quality than the consumer devices used by dermatologists in the exam room, there will undoubtedly be similar characterization requirements that will be needed to support the implementation of advanced visualization and diagnostic aids.
What is currently considered best practice for the characterization of microscope cameras in pathology and how much of this knowledge is incorporated in the current DICOM standards? Are sufficient process and configuration details specifically called out in the DICOM image metadata? Are digitized visible light pathology images integrated with enterprise PACS systems in a manner that can support functions other than diagnosis such as quality assurance? Such precedents could form a roadmap for dermatology as well as for the other visible light specialties.
Is there a standard range of acceptable hue and saturation values for the different stains? What image processing and visualization aids have been developed to aid pathologists? Are they linked to DICOM or are they proprietary vendor tools? Would pathology benefit from a version of the proposed dermatology Imaging Utility Graphical User Interface (IUGUI) to assess the image quality and to facilitate visualization of diagnostic detail on the pathology slides in a manner similar to that done for diagnostic skin images?
It would seem prudent to seek the advice of dermatopathologists to determine if there are any requirements that are particular to dermatologic diagnosis that are not being incorporated in the current DICOM pathology imaging standard. [11-12] With the increase in the quality and accessibility of telepathology, dermatopathology is becoming a distributed service offering universal availability in a timely manner. This transformation to digital dermatopathology brings with it the potential for analytic and visualization tools. More importantly, it brings with it a capacity for quantification that will result in better diagnostics, better diagnoses, and better care.
Teledermatology - The most widely used application in dermatology that actually depends on imaging for diagnosis at the current time is teledermatology. [13] Teledermatology has the potential for delivering scarce specialty care to underserved regions in an economical fashion. This begs the question: why does a much needed service like teledermatology so often fail? Certainly, regulatory and reimbursement issues constitute appreciable impediments[2], but all too often it is the inability to reliably capture diagnostic quality images that is cited as the cause of a program’s demise. Dermatology is telemedicine’s Wednesday’s child. Practically any imaging factor that is troublesome in another telemedicine imaging modality is worse in teledermatology.
While other telemedicine imaging applications almost always constrain the pose of the camera to a single, or small number of set, repeatable imaging environments, teledermatology is forced to contend with either obtaining less than optimal views of the patient and the inconvenience of positioning a tripod in the confines of an examination room, or incurring the image degradation resulting from handheld camera operation. Use of a tripod will require the expense of another set of hands to (re)position the camera while imaging an articulated, deformable target over an appreciable range of scale. The alternative is to incur the inefficiencies and delays of having a single caregiver breaking off working with the patient to adjust the camera. Continuing Wednesday’s woe are image distortion and corruption due to motion and defocus blur, chromatic shifts due to variations in the ambient illumination, and manipulation of the images as a result of edge sharpening, noise reduction, chromatic saturation enhancement and other in-camera signal processing.
These quality issues have import not only for dermatology but also have the potential to degrade diagnostic performance in a number of other specialties (e.g., home telehealth and teledentistry) that also have come to rely on equivalent COTS equipment because of the associated economies. These specialties are in need of the same device assessment and characterization as dermatology. It is not that COTS devices won’t work. It is that the consumer marketplace is continually evolving and is driven by disparate influences whose clinical consequences need to be monitored. Devices in the digital camera marketplace have a sales window of from 14 to 18 months. No camera is perfect and there will be a continuing motivation to upgrade so as to be able to adapt all of a new camera’s capabilities to the needs of teledermatology. In addition, upgrades all too frequently occur because of the reality that digital cameras do not bounce very well.
Beyond simply imaging a lesion for documentation, there is a need in teledermatology to convey the information usually garnered in a physical exam. Ultimately, this distinction will be the motivation for an explicit DICOM teledermatology procedure that builds on some of the ideas in the American Telemedicine Association (ATA) Guidelines [14] and grows them into a comprehensive remote imaging standard. What activities and parameters would such a process need to encompass? While many procedural issues have been outlined in the ATA Teledermatology Guidelines, that document should be considered a first pass, an advocacy that speaks in support of existing practice. It is a document that needs to be extended and made to go deeper into device characterization and to address the complexities of using consumer-grade digital cameras and displays in the clinical setting. The very same aesthetic manipulations that make these cameras attractive to the consumer also enable the economies of scale that make these devices attractive to the clinician. The trouble is that these aesthetic influences may alter images in ways that are not always obvious or in the best interest of the patient.
The ATA Guidelines for Teledermatology provide suggested imaging protocols for Store and Forward (SF) and Real-Time (RT) versions as well as Hybrid (HY) systems that combine both video and high resolution still images. The advantages of SF teledermatology are derived from high quality digital still images acquired by trained staff at the originating site, but with the cost of delayed viewing and the loss of any direct interaction between the physician and the patient or staff. RT teledermatology allows for such interactions at the time of the examination, however, the cost here occurs with the substantially lower image quality available with the transmitted video. The HY approach is an attempt to merge the strengths of SF and RT methods at the cost of purchasing both video and still cameras as well as a means to convey both types of images to the distant site. Work remains to reconcile the ATA Guidelines [14] with the AAD Position Statement on Telemedicine [15] concerning such issues as the latter’s mandate to have a high resolution camera at the originating site or the omission by either to address image quality differences between digital and analog video.
To convey the information required by the remote teledermatologist, a range of patient views is needed. The ATA Guidelines suggest a functional hierarchy of clinical images. SF commonly provides a wide angle patient view, a contextual view that establishes location by including unambiguous anatomical landmarks, and a diagnostic image that provides the highest detail. The patient view provides the remote clinician a sense of the entire patient that is usually unconsciously appreciated through the proximity and interaction of a face-to-face physical exam. Contextual images have utility in that they give both a better impression of the surrounding uninvolved skin for comparison as well as evidence of the anatomical location of the region of interest. This positional evidence is absent from a macro image because of the restricted field of view that is required to provide the resolution necessary for the acquisition of diagnostic detail. Freed from a need to provide such contextual information, the diagnostic image can restrict the field of view to a few centimeters, resulting in pixel projections on the skin that range from 25 to 5 microns. Consider how stable hand position must be to hold a pixel congruent to that size patch of skin even for the 10 to 20 milliseconds the shutter is active (and while pressing the shutter release). However, with the proper technique, tissue detail can be both captured and appreciated at a level of resolution that is ordinarily reserved for the pathology laboratory.
At the present time, there is no requirement in the ATA Guidelines to retain any imaging used in an RT teledermatology session. While it is certainly the case that it may not be necessary to create a video archive of the entire session, it would seem equally clear that some visual record of the clinical evidence should be preserved. One reason for this record would be to assess change in the condition of the patient over time, while another would be to perform due diligence by enabling a quality assurance process that is able to obtain a measure of the distortion present in the transmitted images. [16] In addition, these decisions must address the changing thicket of state and federal regulations that govern the delivery of healthcare using telemedicine.[3] Resistance has, and will occur when these requirements disrupt the current practice patterns and requisition additional time or capital to guard against technical or procedural shortcomings. Nonetheless, concerns over expense and efficiency should, on balance, be tempered by the prime requirement to protect and serve the interests of the patient.
One of the bright sides to the use of consumer-grade cameras is the remarkable string of improvements in functionality that have occurred at the same time as the price of digital cameras has decreased. A good example of this evolution is the Canon EOS 5D Mark II digital SLR camera. It has a 21 Mpixel full frame sensor, a 920,000 pixel LCD, can capture and display HD video (and audio), and will accept Canon’s optical image stabilized family of lenses. It is pretty much a teledermatology imaging system in a box. When the camera is released, the list price will be $2600, but that likely will plummet before long due to competition from new models both from within Canon and from competing devices made by other manufacturers[4] (as has been the pattern with other camera price trajectories). Future improvements in video with HD format and in dynamic range with HDR image processing are sure to follow.
It should be apparent from the discussion herein that, contrary to common opinion, even with the increasing functionality afforded by the latest digital cameras, capturing an accurate diagnostic image of a patient is not simply point and shoot. It is more point and shoot yourself in the foot if you’re not careful, and careful in this case requires a detailed technical and procedural standard. A standard is needed to codify the many parameters and settings that influence the ability to capture an image that conveys all required diagnostic evidence. Specifics of relevant capture foibles and pitfalls supporting this caution will be discussed in the Validation and Characterization section below.
Modalities Waiting in the Wings - There are several imaging modalities that, either because insufficient research and validation studies exist or because of a lack of market interest, are not yet ready for clinical use. In addition, there are other imaging modalities that span disciplines and may more properly be shared between or appended entirely to a different part of the DICOM standard. Many of these dermatologic imaging devices and technologies have appreciable merit and are on the cusp of clinical acceptance. Their clinical and commercial progress is being retarded, in part, by a lack of standards. Adoption of these instruments and techniques by dermatologists will be facilitated by the creation and implementation of standards and the consensus they engender.
The following is a list of dermatologic imaging modalities that potentially could provide various forms of assistance to the diagnostic process. Some of these modalities have appreciable commercial support, while others are entirely products of academic research. All would benefit from more validation work leading to the creation of standards.
Digital dermoscopy: For a very long time, dermatologists have relied upon magnification to increase apprehension of fine detail and the application of wetting agents to improve surface contrast. The packaging of these features along with specialized lighting in dermatoscopes made changes in tissue morphology apparent that previously were not visible to the unaided eye. [17] Improvement in the perception of skin lesions using these devices is due in large part to the virtual elimination of surface specularity. The accessibility of this degree of fine tissue structure has led to the proposal of a number of feature sets that claim to be diagnostic for pigmented lesion diseases, and in particular, for melanoma. Difficulties associated with the existence of multiple feature schemes have been muted appreciably, if not entirely eliminated, by the published AAD 2007 consensus on dermoscopy. [18] The AAD consensus paper provides best practice guidelines for the evaluation and diagnosis of skin tumors using dermoscopy.
A whole host of telemedical and
assistive diagnostic applications are enabled by the ability to attach a
digital camera to most dermatoscopes. The contribution of the consensus paper
that would particularly impact any dermatologic imaging standard is the list of
features diagnostic for pigmented lesions. It would be advisable to take steps
to ensure the quality of the representation of these features in images used
for diagnosis, and to document
appropriate quality measures in the image metadata. Diagnostic concerns related
to image quality can go beyond the characteristics of the capture transformation.
Because of the adaptive capabilities of the human visual system, there are
often differences in the perception of a lesion in the exam room and the
perception of even the best of images of that lesion. While these perceptual
differences are often induced by transformations made by the capture and
display devices, however, perceptual changes also occur when images comprise a
relatively small region in a environment appreciably different than when the lesion
is viewed directly through the dermatoscope. The development of a color
calibration artifact along the lines proposed in the dermatopathology section
above would eliminate much of the chromatic perceptual distortion.
Because of a concern over the uniformity of lesion appearance across
capture devices, there is a need to specify the wavelength spectra and physical
arrangement of all sources of illumination used in the various dermatoscopes. The
influence of factors such as color management, wet or dry contact, and
polarization also need to be addressed. It should be remembered that it is direct
viewing dermoscopy and not the derivative viewing of images or the use of
subsequent analysis that has been sanctioned by the AAD consensus report. While
there is a considerable literature on image-based dermoscopy, there is much
validation that remains to be done.
Assisted diagnosis: The complexity
of diagnostic judgments currently render the performance of completely automated
dermatologic diagnosis insufficiently accurate to be put into the clinical
environment and will likely remain so for some time to come. [19-20] However,
the use of computers to provide assistance to clinicians by extracting
properties of lesions obtained from images in a consistent and quantified
manner has been available for the last twenty years. [21] Some radiological applications have incorporated
a machine-based, automated second opinion in double reading paradigms and have
achieved significant cost savings. In dermatology, these systems are primarily
used for the diagnosis of pigmented lesions and have demonstrated performance
improvements when used by less experienced dermatologists. As with dermoscopy, pigmented
lesions have yielded the biggest diagnostic return, although both imaging
modalities have a growing application base beyond melanocytic lesions.
Assisted diagnosis is conceptually next door to evidence-based medicine
(EBM). It takes the clinical knowledge structure one step further by
introducing a partial automation of diagnostic analysis. Usually the hardware
is composed of a desktop computer that is linked to a digital video (or sometimes
a high resolution still) camera and is arranged so the whole apparatus can move
about on a small lab cart. These platforms provide useful signal and image
processing techniques that are applied to the diagnostic images. However, clinical
adoption has been limited because existing systems remain mostly proprietary
and expensive.
Assisted diagnosis systems do not yet possess enough market share to
have a significant impact on dermatology. Currently, it is a fragmented,
parochial market beset by divergent technologies with varying degrees of
validation. It is another application that would benefit by the implementation
of standards. However, at present, there is no large database of validation
studies supporting any one technique (of which I am aware) around which users
could form a consensus of best practice.
Body mapping: Imaging
is often used to monitor the progression of diseases, of treatments, and of the
subsequent healing process. Body mapping is commonly applied to cases where
there are large numbers (up to hundreds) of discrete lesions. [22-23]
Documentation of pre- and post-surgery conditions or of rashes that
cover large regions of the skin are considered forms of body mapping as well. In
some cases, the images of past states could be used to appreciate and quantify
the progression of a treatment (e.g., for psoriasis) or to follow the change in
the state of a disease through local alterations in a single lesion or subset
of lesions (e.g., for dysplastic nevus syndrome).
The body mapping process is composed of two functions: indexing the
individual lesions so that they can be uniquely identified, and detecting and quantifying
change in individual lesions over time. Both functions are commonly applied
when there is a multitude of lesions; however, there are times when only a
single lesion needs to be monitored. Each function is susceptible to a
different source of error: indexing error – retrieving the wrong lesion for
review, and change error – misinterpreting a change in skin pose, articulation
or deformation as organic change.
As with teledermatology, application of proper acquisition technique is
paramount to the capture of results of diagnostic quality. Indexing errors are usually
the result of too great a density of lesions for the available digital
resolution. Some positional variability can be tolerated but without sufficient
pixel density projected on the skin it is not possible to sustain adequate
accuracy of correspondence when referencing a patch of skin to the patient
coordinate frame of reference. A reasonable tolerance for errors in change
measures also exists. An individual lesion can be correctly categorized even in
the presence of minor distortions. The characterizations obtained from individual
images can be useful in documenting a patient’s condition at a series of different
time points without analytical temporal comparisons being performed.
However, if images are compared over time, a higher imaging standard is
required. When collected in a series with a uniformity of pose that allows
direct comparison, images can be used to measure change, and that change can be
used to modulate the meaning of the observed morphology. When change is used as
a factor to scale the significance of a particular morphology, one which might
not be considered salient in its own right, the source of the change must be
carefully ascertained. Apparent change can occur when the uniformity of capture
pose is not maintained or when there is obscuration of details by hair. Beyond maintaining
adequate camera characterization and calibration, proper control of change
error also requires adequate modeling of the movement of the patient’s skin. Positional
accuracy and apparent shape can be impacted by insufficiently accounting for body
articulation or skin deformation. Maintaining uniformity of capture pose for
lesions over joints or on soft tissue such as genitalia is particularly
problematic. Changes also may be due to alterations in the patient’s weight or
other physiological processes that can either distend or slacken the skin. Change
due to any of these benign processes that is captured with a temporal sequence
of lesion images can be mistakenly interpreted to be the result of some disease
process and then be incorrectly categorized as something more serious than is warranted.
The manner in which the properties of skin alter the benign deformation of
different lesions due to articulation or applied pressure and how that can be
best discriminated from disease progression remains to be properly characterized.
Image databases: Historically,
dermatologic images were created for local use, largely for teaching purposes. Today
with the growth of EBM, medical image databases are being used increasingly as
reference material to support diagnostic decisions. This change in use raises
the issue as to whether image databases should be made compliant with a
standard that requires detailed capture information be available if the images
are going to be used in clinical applications (e.g., [24]). The wide variation in image quality to
be found between and within dermatology image databases speaks to this point
quite clearly. To reduce the variability of any associated diagnostic
decisions, it would seem desirable to require uniform capture conditions (or at
least uniform transformations) and thus avoid spurious influences that could
corrupt skin appearance. At the very least, these databases should provide documentation
for all relevant lighting and camera settings.
In the cases where such information is not made available, the creation
of an IUGUI may be helpful in reducing appearance uncertainty. Not only could
the relevant color management and image distortion parameters be assessed, they
could be normalized to a standard viewing condition. The use of standardized
medical photography labels with reference white patches, skin color strips, and
step edges would go a long way to promoting database uniformity. Incorporating
these requirements into a DICOM standard for visible light skin images would
increase the pool of diagnostic quality images available for evidence-based
dermatology.
Mohs surgery: The use of digital images for
mapping margins can provide an accurate method to determine excision defects. [25-26] The
availability of the excision images in the patient record can also provide
documentation of the procedure. This documentation can help to obtain
appropriate reimbursement from insurers. When images of the surgical defect are
captured, they may also help to protect the surgeons from subsequent litigation.
Development of 3D tools also could allow insertion of the images into an
incremental excision model. Such visualization aids would provide improvements
over current manual methods of tissue section alignment.
Polarization and wavelength
filtering: As described above, polarization can be used
to advantage in dermoscopy, but it also can be used improve the visibility of
skin surface features in normal reflectance images. [27-28] Because
of the enhancing effect perspiration and sebum has on the magnitude of specular
reflection, particularly in the facial region, appreciable diagnostic surface
detail can be obscured. The specularity can largely be removed with a linear
polarizer. The influence of various combinations of polarizers on the source,
on the camera, and ratios of crossed and uncrossed measures should be
investigated, and documented in the image metadata.
Building on the tradition of Wood’s lamp applications, digital UV
imaging can be used to enhance the visibility of melanin in the skin. [29-30]
Digital UV images offer an advantage over the traditional methods in
that the UV images are amenable to quantification and subsequent analysis.
Over the last few years, there has been an improvement in the quality of
IR imaging as well as an appreciable reduction in the cost of these devices. Thermal
images can do a great deal in the documentation of vascular problems and also for
measurement of wound healing. [31-32]
All of these applications based on properties of light whose
interactions with skin need to be properly characterized and validated. If these
studies lead to coverage by a standard, it will help promote a uniformity of
appearance that can aid diagnostic performance independent of the specific
device configurations.
Confocal scanning laser
microscopy: In
vivo confocal microscopy has been shown to aid in the diagnosis of pigmented
lesions thereby demonstrating a performance improvement over dermoscopy. [33-34]
There is an appreciable amount of academic work with this class of
device but there are a limited number of commercial vendors of instruments capable
of in vivo imaging. This is an area where a simple standard documenting parameters
such as in-plane and axial resolution and data configurations such as image stack
layout would help advance this promising technology. Given the change in
confocal image properties with tissue depth, this is an application where an
IUGUI specialized for confocal imaging could go a long way in improving
diagnostic utility.
Optical coherence tomography: OCT
offers a non-invasive technique of imaging fine, micrometer-level detail of the
skin. [35-36]
This technique is thus able to distinguish morphological features that support
diagnostic discriminations. The technology is largely a research product with commercial
devices just becoming available. It is another case where being able to
incorporate appropriate device documentation in an imaging standard would help
advance adoption of the technology.
The following modalities have application to dermatology but each also
has a significant overlap with the purview of an existing DICOM Working Group.
Wound healing: What
is the relationship between cases that fall under the description of wound
healing and dermatology? There is a broad spectrum of wound cases (viz. [5]) that disrupt the normal activity of the
skin – compound fractures, punctures,
cuts, abrasions, chemical and thermal burns, blunt force trauma, surgical
reconstruction for treatment of disease or resulting from combat injuries, bed
sores in long-term care, ulcers due to circulatory problems, any of the
hundreds of diseases that disrupt the communication of the normal operation of
the blood supply to the skin, insect bites, and viral or bacteriological
infections. All cause substantial breakdowns in the barrier function of the
skin. These events literally remove the usual protections provided by the skin
and open up the body to external assault. Wound healing is associated with many
causes that are all linked together by a debility resulting from the breach of
the skin’s defenses and the consequential onset of infection and loss of
utility.
Images that document wound injuries are different from many dermatologic
images in that they often contain a substantial 3D component on the skin
surface that is of clinical interest. [37-38] 3D measures of wound extent are helpful both
to assess the initial degree (and depth) of injury and as a way to track the
repair process and the restoration of the barrier function. While much of this
image data is gathered in a qualitative fashion with the use of tangential
lighting and with a pose that enhances shadows and relief, it is certainly
possible to use the information in wound images with varying pose to
reconstruct a 3D map. In this, the efforts of WG-17 (3D) may provide an
existing framework.
Ultrasound: Ultrasound has been used as a pre-surgical
tool to non-invasively determine the extent of a lesion. [39-40]
Other applications of ultrasound such as in wound healing have also been
attempted. The increasing availability of small, portable ultrasound devices
could dramatically increase their use in dermatology. Whether these
dermatologic images can be accommodated by the properties of the existing WG-12
(Ultrasound) standard needs to be determined.
Video: While the use of high resolution digital
still images is common in dermatology, there are video applications for both
body mapping and RT teledermatology. These systems need to provide more than
teleconferencing. There is diagnostic information that needs to be captured and
preserved by a medium that has limited capacity. [16] To overcome these limitations, video
sensors have incorporated a much wider range of configurations than still image
devices. Very few of these innovations have been validated in clinical
applications. The relation between these imaging requirements and the
capabilities of video communications protocols used in DICOM need to be determined
and validated.
Compression: Several
studies have shown that JPEG2000 is advantageous for dermatologic imaging. [41-42] The
ability to enjoy appreciable compression savings without incurring visible
artifacts becomes more important as color images from consumer cameras cross
the 50 megabyte level. The work of WG-4 (Compression) will have application
here. Of even greater relevance should be the work of WG-26 (Pathology) in
reducing the file size of visible light color images while preserving diagnostic
detail.
Cosmetic surgery: Cosmetic
dermatology has greatly expanded over the last two decades. The use of color
digital images to document procedures from skin peels to reconstructive surgery
need to be characterized and validated. [43-44] In addition, documentation of the use of 3D
tools for predicted cosmetic appearance, surgical planning, and for the
objective measurement of results would benefit from the information structures
developed by WG-17 (3D). In particular, a standard that aids in the documentation
of procedures and results would be beneficial in resolving litigation.
Clinical trials: Historically in clinical trials, disease
severity has been assessed through the creation of scales that were applied by
human raters. [45] Beyond
issues with accuracy and consistency, these labor-intensive methods have been
costly to implement. Increasingly, measures based on skin imaging have been
used that are often supplemented with computer image analysis. [46] As
resolution improves and the costs go down, COTS digital cameras are being used
more often to capture evidence in support of clinical trials in dermatology. These
devices are subject to all the quality concerns described elsewhere in this
document. The use of these devices needs to be integrated with the efforts of WG-18
(Clinical Trials and Education), as well as the substantial sensor and display
characterization effort at the FDA. [47]
Not all of the above modalities will appropriate activities to be
covered by the dermatologic imaging standard. Nonetheless, it will be the case
that some, if not most, of these modalities will become productive utilities
for dermatology in the future and would certainly benefit from some form of
standardization and regulatory structure.
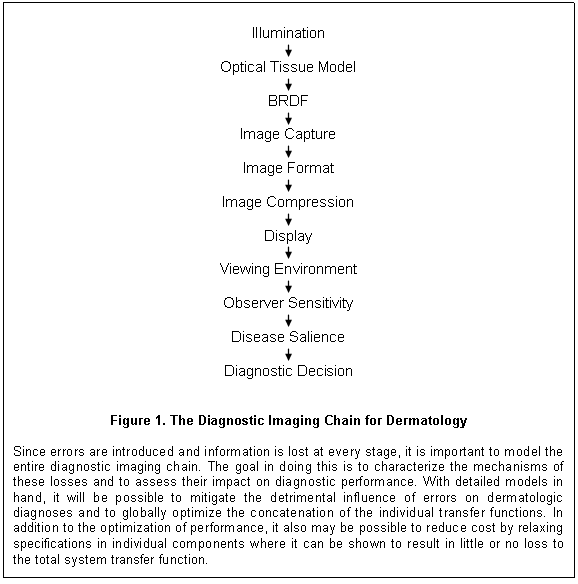
Characterization and Validation - In the final analysis, it
doesn’t matter why or how diagnostic information is lost (camera motion, glare
on the monitor, or the need of a prescription for new eyeglasses). If necessary
information is not available for diagnostic decision making, more errors will
be made. It is crucial, therefore, to consider all the influences that impact
the creation of an image and its subsequent viewing. To this end, a detailed
description of the proposed imaging chain for dermatologic diagnosis using consumer-grade
single-sensor (CCD/CMOS) color cameras and commercial LCDs is presented in this
section. The settings, the procedures and the utilities associated with this
model of diagnostic decision making constitute an implicit validation to-do
list for dermatologic imaging. Following this outline is a discussion of the research
and resources that will be required to bring all the elements of the Diagnostic
Imaging Chain Model into clinical practice. Enough detail will be presented to
capture the form and flow of the diagnostic process, but the inclusion of all
the relevant parameterization would dilute the organizational focus of this
whitepaper.
Illumination / Tissue Optics / BRDF: The Diagnostic Imaging Chain for Dermatology (figure 1) begins with the characteristics of the illumination. The sources can be diffuse and uncontrolled as with the ambient lighting in an exam room, or more local and controlled as with that of a dermatoscope. The intensity, spatial distribution, wavelength spectrum, and polarization of the illumination all influence the extent to which the light is distributed within the skin or is reflected off of its surface. The anatomy of the skin, often distorted by disease, determines the distribution of transitions in the index of refraction of tissue, the types of chromophores, and the sizes of scattering media – all of which vary with anatomical location, gender, skin color and age. Through refraction, cell membranes and other tissue structures each introduce their own individually small contribution to the deviation of the light as it travels through the skin. In addition, the incident light interacts with chromophores and scatterers in a wavelength-dependent fashion, further increasing the complexity of its distribution. The net result is a transformation of the incident illumination into the pattern of backscatter that emerges from the skin.
Merged with this intrinsic reflectance distribution is the portion of the incident light that is directly reflected off of the skin, the surface specularities. The specular reflectance component is a function of the angle of incidence, the pattern of microrelief of the stratum corneum surface, and the polarization distribution and wavelength spectrum of the incident illumination. Through a combination of subsurface light propagation and the surface specularity, the interaction of light and tissue forms what we perceive as the skin’s appearance. The filtering and redistribution of the illumination by the skin is more formally characterized by its Bidirectional Reflectance Distribution Function (BRDF) and its subsurface variants. [48]
Image Capture: The next section of the imaging chain involves the
transformation of the intricate mixture of scattered and reflected light into a
two-dimensional numeric array by a digital camera. The first link in this part
of the imaging chain is defined by the entrance pupil of the camera lens. The
size of this opening determines the segment of the light distribution leaving
each point of the skin (the local BRDF) that will be funneled into the
lens and ultimately focused on the
sensor array. As indicated above, the captured distribution is an angular subset
of a complex pattern that itself is a function of the angle of the incident
light and the local orientation of the skin’s microrelief facets. The collected
segment of the BRDF is a combination of the intrinsic backscatter and specular
reflectance of each measurement point plus a portion of the light incident at
varying spatial offsets that through light transport in the tissue that also emerges
at the current measurement point.
The ability of most (even consumer) lenses to focus the spectrum of wavelengths to a point on the sensor is quite good, but none are perfect. Even if they were, there would likely still be errors in making the nonplanar skin surface congruent with the sensor plane. The result being that the photons which emerge from a single location on the skin are not resolved to a single point but are distributed among proximal photosites. Adding to this spread is the consideration that as the light approaches the single-array color sensor it passes through two filters that are usually installed sandwiched together, a birefringent filter and an IR-cut filter. The first reduces chromatic artifacts by ensuring that all incident light is spread horizontally enough to cover the two pixel column width found in most color filter arrays (CFAs), which are themselves a repeating mosaic of wavelength filters that selectively reduce the photon catch at each photosite. The cost incurred to lessen these chromatic artifacts is a reduction in the obtained horizontal spatial frequency sensitivity. The second filter ensures that the appreciable native sensitivity of the sensor to the near-IR wavelengths is blocked so that only contributions of the visible region of the electromagnetic spectrum are collected. Finally, with passage of the light through the camera lens, sensor microlenses and CFA, and around the opaque active elements of the photosites, information about the imaged scene passes from photons to electrons.
Image Format: Once contained in an array of potential wells, the resulting charges (minus a few from photons that were lost en route through the lens and sensor, and plus a few electrons generated by noise processes in the sensor) are proportional to the light leaving the small patch of the skin’s appearance being measured. The process of representation and abstraction of the skin’s reflectance begins with the charge in each well being transformed into a number. Digitization is performed by dividing the range of charge held in each well into a set (usually a power of 2) of contiguous intervals and assigning values to each interval an incremental sequence. The size of a quantization level is commonly made to be slightly larger than the magnitude of the aforementioned noise. The range of the digital transformation depends on the sensitivity of the sensor and can encompass from 1024 to 65536 quantization intervals in currently available consumer-grade cameras.
At this stage, many manipulations can be performed on the signal – some are optional, some are not. For example, the input signal can be made to undergo a nonlinear transformation that approximates the brightness perception conversion of the human visual system, or, alternatively, the transformation can be kept linear. Also, the captured pattern of filtered light can be converted to a full tristimulus representation at each photosite by interpolating the values of each channel of the color mosaic. Alternately for increasingly many camera models, a representation very close to a digitally sampled version of the latent image can be saved in the form it was captured by the sensor (RAW format). In the former case, signal processing in the camera efficiently provides the conversion (at the cost of tripling the size of the data while adding no new information). In the latter case, the file size is commensurate with the original information, however, this choice incurs the cost of doing the tristimulus conversion in the future on a computer external to the camera. The external conversion often requires the incorporation of very detailed knowledge specific to the sensor to produce high quality, true color conversions. There are many other manipulations which largely have to do with image quality issues (e.g., sharpness and contrast enhancement, color management, and noise reduction) that may take different forms, often varying significantly across camera models. Some of these manipulations are helpful to the diagnostic process, some are not.
The next link in the imaging chain involves configuring the quantized image data into a file format for storage and transmission. Apart from the unvarnished (RAW) representation described above, file format conversion usually involves the loss of information. This loss is the result of reducing the number of bits per color channel from that which was present at capture. Capturing more bits than are used for display allows digital cameras degrees of representational freedom that are analogous to the older ISO film sensitivity ratings. With digital capture, a different mechanism is employed to deliver the same shift in the light sensitivity of the captured image. By being able to apply any of a range of speed/aperture reciprocity curves, the image content can be shifted to different quantization levels. Lower quantization levels require less light but incur more noise – just like using film with a higher ISO rating. By applying these shifts in quantization levels, better sensors with greater overall light sensitivity and less intrinsic noise allow a greater range of luminous environments to be imaged.
Commonly in consumer cameras, 8 bits (256 intervals) are used to encode the number of quantization levels used for each color channel at each tristimulus pixel. Most of the time, this quantization resolution is sufficient to represent color images of the skin without incurring too much loss of detail in the shadows or too much clipping of the highlights and still not be so coarse as to induce perceptible banding. There are at least three reasons, however, for more bits to be retained in an image representation. First, in a growing number of applications, it has been found to be useful to double the size of the representation to 16 bits (65,536 intervals) per color channel so as to minimize the artifacts induced by computational round off errors that can occur in image processing subsequent to capture. Second, quantization errors may be more readily apparent in many high-end displays used in medical and graphics applications due to their greater uniformity, lower noise levels and generally increased image quality. The representations produced by these devices are frequently good enough to require more than 8 bits per channel to avoid perceived artifacts such as banding in shallow luminance or chromatic gradients. Finally, minimizing the loss of information during image capture and formatting provides greater diagnostic flexibility. Data can be migrated to more powerful image processing environments external to the camera at a later date as considerations of care dictate. With the data represented by formats such as RAW, all visualization, display and analytical options can be retained.
Image Compression: As image structure increases in size, there will be greater pressure to reduce storage and transmission costs by (often irreversibly) reducing file size and discarding parts of the image representation. In justifying this reduction, various assertions are made about the utility of the discarded information to the task such as its characterization as noise and its visibility to the observer. Establishing what trades to make poses an interesting circularity problem. If images are compressed prior to the formation of a diagnosis, how is it possible to determine what can be discarded from an image without knowing what detail needs to be represented and how is this known without knowing the correct diagnosis? How can an applied compression be considered undamaging to essential diagnostic features if it is not known what is essential? Even after a diagnosis is made, how can the ability to review an image and to amend the diagnostic decision be preserved?
There are hundreds of named dermatologic diseases with varying types of detail that must be accommodated. A conservative approach would require each image to retain the capacity to accurately represent the entire set of these diagnostic features. Is it possible to comprehensively represent the set of all diagnostic features while sufficiently reducing file size and yet not corrupting any of the features by inappropriate economization? This assessment is not straightforward. The effects of corruption lie on a slippery slope. Compression damage doesn’t necessarily make a given diagnostic decision impossible to make. The diagnostic decision may just become more difficult, the evidence less compelling, the conclusion presented with reduced confidence.
Much of color image compression research has been concerned with the management of a color palette selected for aesthetics, not diagnosis. The goal in these studies was to make changes in color values so as to reduce the file size required to represent the image while avoiding displeasing artifacts. What is of concern here is how these adjustments affect the diagnostic features required for dermatologic diagnosis. What needs to be preserved changes whether it is erythema for PASI scores in a clinical trial or a blue veil related to the C in the ABCD rule for melanoma detection that is being clinically assessed. It is not clear how easy it is to degrade different diagnostic features (spatial, luminance, or chromatic) especially with the need to be robust in a variety of contexts (e.g., anatomical location, skin color, and age). With some compression algorithms, changes in the pixel color values are image specific. Such variations in color change can be particularly problematic for algorithmic assessment when applied to lossy color images.
Validation research will be needed to test the underlying assumptions of diagnostic color image compression, not only to determine how they affect physicians’ diagnostic performance but also to establish how they interact with analytic utilities subsequently applied to the lossy (compressed) images. Once formatted (and potentially compressed), the images can then be stored, retrieved, and transmitted to other applications.
Image Metadata: When a diagnostic system must accommodate a variety of capture devices, it is essential to retain a full description of the genesis of each image. Even when there is a uniformity of devices, the clinical capture environments – different exam rooms, surgeries or patients out in the wards – will alter the appearance of the obtained images rendering comparisons over time or the diagnostic analysis of a single image difficult. The inclusion of appropriate metadata (data that describes other data) will allow an image to be reconstituted on any display or used for any analysis without placing rigorous a priori strictures on its formation. Metadata can be stored in a database that mirrors that of the image archive, can be stored in a separate file that accompanies each of the images, or, more commonly, can be written as part of the image file itself.
Several issues remain to be resolved between dermatology and DICOM with respect to what properties are required to document color images for use in dermatologic diagnosis. The availability of this data frequently takes on added importance in situations where these images constitute a primary source of diagnostic information. Currently, Secondary Capture (SC) and Visible Light (VL) Information Object Definitions (IODs) are being used as major conduits for the incorporation of color images in dermatologic (and other) medical archives, although largely in an ad hoc fashion. This archiving capability has been built on existing utilities that were created for the acquisition and display of color images for secondary applications, noncritical uses that service the majority of the supported DICOM clinical specialties in a variety of functions. The digital color images being incorporated in the medical record have been captured predominantly with consumer-grade devices and the documentation of even their basic properties are confined to the limited capabilities afforded by VL, SC and also by the Color Softcopy Presentation State (CSPS, supplement 100) IODs. The flexibility built into these modalities allows some of the structural camera parameters to be accommodated by the existing VL, SC, or CSPS Service-Object Pairs (SOPs) – if not exactly, then with slight modification. In practice, however, the VL and SC modalities are used simply as backdoors to get color images that were captured by consumer cameras into the medical record and to allow them to be viewed in a DICOM display environment. The barest amount of image properties are commonly provided – the number of rows and columns, the aspect ratio of the pixels, and, occasionally, a choice of rendering intent.
While it is legitimate to adapt existing SOP classes to other uses, the question remains as to whether such a minimal coverage of the camera settings sufficiently preserves the configuration of the capture process to ensure that these images are able to uphold the intended clinical purpose. Barring entry errors, representation of the structural image information is largely without dispute, although the consequences of adjustments to the basic CFA matrix format or spatial variations in pixel layout made by many manufacturers need to be individually examined (e.g., replacing the Bayer pattern with four color filters or shifting the green filter mosaic by half a pixel to improve spatial resolution in some video sensors). It is the representation and regulation of analytic information, information that controls and assesses the quality of the image and its clinical utility, that is often more contentious. It is this information that is needed to support the complex processes that maintain the integrity of the Diagnostic Imaging Chain for Dermatology. These values help define the functions required by quality assurance mechanisms, color management utilities, and image quality assessment. Consider as an example of assessment issues the use of ICC profiles to control the presentation of color in images that are being used as a primary source of diagnostic information. What needs to be documented in the image metadata to ensure that when adjusting the image for variations in the available color gamut (e.g., selecting a rendering intent) there are no changes in the classification of perceived diagnostic features when viewed by the clinician or, alternatively, to ensure that there are no qualitative differences in the outcome of computations performed on the adjusted pixels?
Even with some capacity for functional image documentation in existing SOPs, there will nonetheless be a need for additional assessment protocols such as for motion and focus blur measurement and to address other in-camera image processing that have been found to alter dermatologic image quality. More issues can be raised over the diagnostic utility of these incompletely documented images, not in the least because of the variety of the consumer devices that are used to produce the images, the associated uncontrolled pose and lighting conditions in the exam room, and the uncertain procedures, conditions and settings used for capture. It still remains to be determined midst all this uncertainty how to demonstrate a secure relation between the utility of the informative content that is extracted from the diagnostic images and the objective quality of those images that is affected by the various components of the Diagnostic Imaging Chain for Dermatology. Determination of the optimal configuration will require validation research that integrates influences from across the entire imaging chain. While portions of this undertaking such as the capture optics and reflectance sampling are relatively straightforward, much of the rest, in particular the optical tissue models and the human psychophysics, can be decidedly arcane. Some balance will need to be found in configuring the contents of the metadata somewhere between including just the basic structural image settings and incorporating a complete parameterization of the model.
While much of the specifics of what information needs to be retained with each DICOM image wrapper still remains to be determined, the present reliance on information that is made available by a range of cameras and a range of formats (e.g., lossy JPEG, lossless TIFF, RAW) on a voluntary basis without a standard is a decidedly uncertain enterprise. A variety of manufacturer-specific methods of incorporating capture settings in the image header are available, but none have even come close to becoming de facto standards. Metadata information may be lost from these images as a result of the lack of standards in circumstances where additional image manipulations are applied, or sometimes just by the images being copied. A more secure DICOM-specified metadata mechanism to regulate color image capture and display will be required.
There is also the concern that without explicit guidance on image metadata the vendors may opt for minimal implementations in diagnostic applications that do not take advantage of even the few avenues open to them to document the pedigree of an image by recording the applied parameter options. This shortsightedness will cause problems in the future. Populating EHRs with such under-documented images will make it difficult to implement more sophisticated diagnostic assistance functions or quality assurance monitoring, may subsequently require a costly cleanup of the medical databases, and could retard the gains in performance and reductions in costs that otherwise could be achieved. In addition, laissez faire incorporation of images into the medical record promotes a decision process that is burdened with an uncertainty of evidence and leaves open questions of adequacy. This degree of doubt and its impact on diagnostic confidence is unlikely to be in the patients’ best medical interest. No images should be placed in a medical record that do not have an explicitly defined contribution to the diagnostic imaging chain and that do not have a documented parameterization history to support each instance of that use.[5] Off-label use of an image should always be an available option that can be exercised as a matter of clinical judgment, but this decision should likewise be clearly documented in the medical record.
Display: The next transfer function in the diagnostic imaging chain is image display. [49-50] When standards are proposed and cost is not a constraining factor, it is easy to imagine ideal viewing conditions with muted, indirect lighting suffusing large, calibrated, uniform, high resolution, medical-quality displays that possess high dynamic range and low noise while displaying color-corrected images. With consumer-grade displays in dermatologic hands, all these properties are not so ideal and can be expected to come much closer to impacting diagnostic performance. Foremost among the concerns over the use of consumer-grade displays for diagnosis are spatial resolution, dynamic range, luminance uniformity, interference from ambient illumination, and color management coordination among the digital cameras, images, and displays.
With respect to the effect of display resolution, there is a trade between dermatologic image detail and subject context when selecting what part of usually much larger images to present. To apprehend the finest capture detail, only about 2 to 5 percent of an image can be seen at one time in the LCDs of current digital cameras, only about 5 to 20 percent using a megapixel desktop display. These small apertures provide only a limited context, a restricted sense of the patient compared to what was seen through the viewfinder and captured by the camera. Validation studies will be required to determine the acceptable relations between the scale of the required diagnostic information and the resolution of the image and of the display.
Consumer-grade displays usually possess a smaller dynamic range, more vignetting and less uniformity in general across the screen than displays designed specifically for medical applications. These limitations can result in banding in the appearance of shallow luminance gradients and greater difficulty in assessing changes in tissue properties over the viewing area. While there is much that has been done in characterizing grayscale performance for radiological applications, much remains to be done to characterize displays where color is a primary source of diagnostic information. The limited ability to maintain uniformity of chromatic representations across different displays further reduces the accuracy of dermatologic diagnosis where so much depends on subtle changes in color as a differentiating cue. Can tristimulus white balance mechanisms provide sufficient uniformity in digital capture or is some form of ICC profiling necessary? If ICC profiles are required to maintain an adequate level of diagnostic performance across devices, what sort of controls on the extent of color gamuts and rendering intent will be needed so that the native chromatic features remain undistorted and do not deleteriously affect diagnostic search or temporal comparisons?
Viewing Environment: A growing issue for medical image viewing is the current trend to use devices that trade accuracy for portability and convenience. Mobility compounds problems with the appearance of diagnostic images by reducing visibility due to the presence of glare common with anywhere viewing. Additional problems occur from presenting limited detail due to small screens whose major virtue is that they come on compact devices that are convenient to carry about the clinic and so do not encumber workflow. This issue has not been unexamined. The parameters, settings, and test utilities used in radiology should be explored further to see to what extent they can be effective with dermatologic imaging applications. Some clinical subspecialties, such as mammography, have developed detailed specifications related to the acquisition of diagnostic features, while for other domains requirements remain quite structural and generic, thereby raising concerns. Not all issues pertinent to dermatologic imaging have been resolved either. It is unclear what provision has been made for the color management of these modest LCDs? New research will be required to determine what needs to be done to measure the effects of the luminous environment (its intensity, structure, and chromaticity) on the perception of electronic content being displayed on a mobile monitor that was designed to be aesthetically pleasing. The uncontrolled viewing environments inherent with the move toward unrestricted portability raise other issues not heretofore addressed by radiology concerning the relation between color management and influences to appearance and detectability brought about by the effects of those variations on the perceptions of clinicians.
One concern about handheld, anywhere viewing is the potential for changing contextual conditions. There is evidence that a moving frame of reference such as with a handheld device may make perceptual degradations less easy to appreciate. [53] How robust is a handheld device as a medical display when it comes to induced motion blur? When assessing tracking performance of a binocular robot, a video of the target from one of the video cameras appeared to be of much higher quality in the context as captured containing tracking error than when viewed with the same sequence but with the target stabilized in the field of view. It is possible that a human observer will be even less aware of a loss in sensitivity to detail because of proprioception associated with the handheld control of the position of the device providing even more motion context. While moving from a radiology reading room to an exam room itself raises concerns, at least a primary medical monitor on a desktop can be positioned and calibrated in a stabile configuration.
What can be expected from diagnostic performance when the viewing conditions of a radiology reading room are replaced with those of a dermatology clinic exam room? Should the conditions present in a dermatology exam room be considered as poor as those of anywhere viewing, and for much the same reasons? Some reports downplay the effects of glare on diagnostic performance. [51-52] Do these reports of glare having a smaller effect on diagnostic performance than was previously thought mean radiologists will be giving up their reading rooms for Blackberries and iPhones? Which approach is correct, the one based on the earlier studies that called for medical-grade displays and controlled viewing conditions or the alternative held by a growing number of users who believe that many of the current generation of ‘smart’ devices are adequate to display diagnostic images? When a large body of long-standing results is overturned in favor of a novel paradigm that offers convenience and profit, it would be comforting to have an independent line of research based on physics and physiology provide corroboration of the new findings. Providing such a reality check is one of the benefits of creating the Diagnostic Imaging Chain Model for dermatology.
Observer Sensitivity: What should be done to assure the adequate
visual sensitivity of the clinician given the demands of the images, the
display, the viewing environment and the current state of the observer? It
would be well to determine and to document the sensitivity of the observer that
is available at the time the diagnostic decision is being made. Current data on
clinician performance need to be viewed in the context of the
A separate issue requiring validation involves determining the clinician’s relative visual sensitivity to all of the various forms of diagnostic detail. Can this information be captured by a set of basic and easy to implement visual tests that can be deployed on displays in the clinic? Some vision testing will need to be done daily, some annually, some perhaps only once, but at whatever schedule, it is a recalibration that should reduce the variability of performance estimates. Given that salience is a major contributor to diagnostic visual performance, it would be helpful if the composition of the diagnostic features could be related back to the structure of the simple vision tests proposed above. This notion gains in significance when considered in the context of the wide range of devices and viewing environments that are now allowed for primary reading of diagnostic images. Performance assessments that increase self-awareness of diagnostic capabilities should be considered appropriate adjuncts to clinical decision support systems. Again, validation studies will be required to bring all of this into the clinic.
Operational testing of clinicians in the workplace will not only ensure that diagnostic performance is within acceptable limits during the course of a normal workday. It also can be used to ensure that debilitating conditions (long hours, illness, age) do not impact diagnostic performance within the existing work environment. It is not that fatigue is necessarily a common cause of misdiagnosis, it is more that misdiagnosis due to misapprehension may be relatively easy to avoid with visual performance testing and the associated heightened awareness. These tests could be applied as a cost-effective quality assurance measure.
Disease Salience: The final section of the Diagnostic Imaging Chain for Dermatology concerns the formation of a diagnosis based on the search for and the apprehension of diagnostic disease detail. [54-55] Something is commonly thought of as salient if it is conspicuous or prominent. In the context of a physical exam, however, an appropriate interpretation of salient is closer to the description of properties of the skin which capture the attention of the clinician because they distinguish the currently observed tissue as potentially other than benign. This recognition is not adequately described by a linear process. In the clinical context, features are not necessarily, or even often, salient because of an objective sensory magnitude that is appreciable. These features are salient to the clinician because of years of training and experience. They stand out because of their membership in a set of patterns that are actively being sought by a tuned perceptual system of heightened sensitivity. In practice, salient features exhibit a range of objective magnitudes that cover the gamut of detection and discrimination of the constituent image properties. While some malignant pigmented lesions manifest exceptional contrast, others, such as amelanotic melanoma, present with no more contrast than an ordinary blemish. This variability is an appreciable part of what often makes visual medical diagnosis so difficult. A feature is salient more because it is, sometimes barely, sufficient to attract attention than because it so markedly stands out along some basic sensory dimension. Salience allows a feature or features to further the diagnostic process because of the attached diagnostic relevance. The observation of salient features is the trigger that moves diagnostic search to a second, more focused, stage of visual interpretation.
While salient features possess definable visual properties that are distinct from normal, the characteristics of adjacent healthy skin and individual visual sensitivity define the observer baseline. It often requires more than the contrast of salience and baseline to trigger diagnostic interest. Often interest is magnified by an observation that is not necessarily diagnostic on its own, but is unusual in the current context. It could be a benign pattern but with a property (contrast, color, size) that out of the norm or it could be two entirely normal patterns not commonly found in juxtaposition. The creation of a compendium of dermatologic disease structures would be helpful in testing the performance of the Diagnostic Imaging Chain Model. Rather than creating yet another hierarchical listing of the hundreds of named dermatologic diseases and attaching a set of named features to each, a comprehensive collection of visible tissue patterns could be created consistent with disease morphology. Beyond offering dermatology residents a more structured exposure to visual diagnostic features than what randomly passes through the clinic door, these explicitly defined patterns could be used by algorithms to search the images for regions that match diagnostic feature definitions or, alternatively, could be directed to regions of interest by the clinician. The diagnostic feature relations could be based on data from resources such as the ICD-10 taxonomy, the AAD’s DermLex, or other open dermatology databases. It would also be important to define equivalently complex structures to represent the null disease, benign skin, in all its variations.
Such a collection of visual structures would be conducive to the creation of the assisted formation of a differential diagnosis. Beyond verbal descriptions, visual templates or 3D structures, it may be possible to achieve robust detection of diagnostic features through the application of more flexible techniques such as the bag of features methods used in computer vision to accommodate variations in spatial phase. [56] The detection process could then be initiated by a utility that extracted feature candidates from the various capture modalities (e.g., surface imaging, dermoscopy, confocal, histology) and assigned modifiers based on age, gender, weight, skin color and anatomical location. It would then be possible to form an evaluation of the likelihood of each disease candidate. Implementing these operations should be easier with plastic descriptions of features than with models built using a more rigid spatial phase structure. With such flexible descriptions it should be possible to attain a more comprehensive set of disease candidates for the differential. Thresholds could be modified and used to search for features not yet found but consistent with the proposed disease candidates. Advantage may also be gained by factoring in as modifiers other information from the patient history or exam notes which could used as additional confidence measures. Defining and extracting disease salience is the key to the diagnostic decision process.
Diagnostic Decision: It is likely that the diagnostic decision is not a simple detection task but depends rather on a battery of cognitive abilities whose capabilities need to be assessed and factored into the imaging chain (e.g., [57]). In some cases, the evidence is subtle and the process is one of detection. When an anomaly is observed in the skin, what methods are available to the clinician to determine whether it is a normal variation of benign tissue or an early manifestation of the onset of a disease? In other cases, when the rash or lesion is manifest, the decision process is one of the discrimination of differences present in an evident pattern. What techniques do clinicians apply then to find specific patterns diagnostic of a disease in already distinct, but quite possibly benign, skin patterns? What is the contribution of focus of attention and how is the evidence collected during diagnostic search built up over time? Does the detection of one diagnostic feature lead to a search for a specific other (or others)? What models of this diagnostic decision process currently exist for dermatology?
It may be possible to construct a model of the detection and discrimination process that could both aid in the measurement of clinical performance and contribute to the formation of a correct diagnosis. Could such a decision model gain insights from across specialties? One source of insight into the diagnostic process that might contribute to the design of such a model would be to present clinicians with cases (either face-to-face or from images) and ask them to verbalize (or write down) their examination thought processes. Investigations of this kind will help define the process of forming a diagnostic decision and will explicate how that process depends on and interacts with the training and experience of the clinician. Increasingly, research also will help integrate the decision support mechanisms based on EBM and other, more explicit, diagnostic visualization aids that build on and assist in the accumulation of patient-specific diagnostic information obtained during search.
Validation: The question now is what is required to bring all of this structure into clinical practice? First and foremost is the need to provide the research in dermatologic imaging that addresses the issues raised above, and then to validate these results in a clinical setting with consumer-grade devices. Facilities will be required for the testing and characterization of cameras and displays as well as the construction of computer simulations of the imaging chain based on these measures. Specifically, there will be a need to create a set of dermatology-based test targets to objectively assess the properties of illumination, capture, compression and display that impact the retention or loss of diagnostic detail. The ability to test all of these areas with a focus on the processing of the required diagnostic detail rather than on the properties of specific devices is important given the rapid turnover of both equipment classes and manufacturers that has occurred in the diagnostic imaging marketplace over the last decade, and that will likely continue in the future. Finally, once the Diagnostic Imaging Chain Model has been defined, it will be important to conduct experiments that test the proposed parameterizations on both clinical and general subject populations.
It will not be sufficient simply to characterize and implement the sequence of transfer functions once and expect that this will ensure the accuracy of the imaging chain representation under all conditions. To the extent that the changes in individual components of the imaging chain that are part of the clinical workflow have the potential to alter the representation of the intrinsic reflectance of the skin and its subsequent processing during capture, storage, display or viewing, the specific details of the acquisition and processing of diagnostic images need to accompany each image file so as to ensure proper interpretation subsequent applications. It will be necessary to design a compact representation of the imaging chain that can be incorporated in the image metadata so that its representation is valid across all devices conformant to DICOM. In addition, it will be beneficial to design an IUGUI that will facilitate visualization of diagnostic detail given the existing morphology, the capture transform, the display transform, and the sensitivity of the observer. The availability of image-specific model parameterizations will go a long way toward enabling these diagnostic utilities. Even a cursory look at the latest radiology journals will bear evidence of the benefits that accrue to the digital promise fulfilled. There is a growing body of work showing how much digitally-enabled visualization can enhance diagnosis. How support might be obtained for the validation of dermatologic imaging is described in the next section.
Business Model - Unlike the earlier successful DICOM standards efforts, there are no market incentives associated with dermatology that can be derived from capital equipment expenditures. Whereas many of the other clinical specialties can count on the resources of several large medical device corporations to provide the extensive corpus of research required to validate devices such as multimillion dollar scanners and their associated algorithms, the capital investment required for a dermatologic procedure is more along the lines of a disposable scalpel. As a result, no way has been found to underwrite the needed research and validation for the visible light imaging described above. Working Group 19, therefore, must not only define the information structures that will be the underpinnings of a dermatologic communications protocol, it must also take up the mantle to both specify and validate the values that populate those dermatologic imaging information structures – for no one else has, or likely will.
While it may not be possible to rely upon consumer camera and display device manufacturers to take on the responsibility for validating dermatologic imaging procedures and devices, it may be possible to garner sufficient support, both financial and advisory, from dermatological societies and from corporate stakeholders to accomplish the task. While they have not gone out and completed this endeavor on their own, it is clearly in their interest to see it done. There are two factors that might have the potential to motivate these affiliations. First, there is the need for complete coverage of all specialties in enterprise healthcare systems for the efficiencies resulting from the digitization of medicine to be realized. Second, there is the potential for the validation of functions for use in dermatology to have a broader utility in medicine and even to extend into more general digital imaging applications.
Over the last quarter century, digital imaging has grown to become a fundamental component of healthcare IT. However, it should be recognized that imaging interacts with many aspects of the digital healthcare environment other than for diagnosis. Coding, billing, quality assurance, EHRs, PHRs, and PACS systems – all have a need to obtain information from, or to pass information to, dermatologic imaging resources. A decade ago, this degree of integration was only a promise. Today it is the target of regulatory mandates. A fragmented or incomplete healthcare IT coverage will retain inefficiencies that raise costs. It is in the interest of every healthcare provider and medical device supplier involved to create a complete electronic healthcare system, one where all aspects of all specialties in the enterprise can be accessed, monitored, and tuned to improve performance. [1-3]
A consulting report on the status of medical technology has described radiology, nuclear medicine and cardiology as the most advanced specialties in the incorporation of digital imaging management while oncology, endoscopy and pathology were considered to be at the beginning stages of using this enterprise PACS technology. Dermatology and the other visible-light-dependant specialties were not even mentioned. But they will be. Not because their bandwidth requirements will exceed all the other specialties as will pathology’s, but they will because of their high utility-to-cost ratio afforded both by the economics of volume sales in the consumer imaging market and by the new advances in computational photography. These low-end consumer products have a better fit to the emerging paradigms of distributed healthcare delivery than those used by dermatology’s high-end colleagues and it is on the innovative use of this technology that much of the predicted savings depend. While home healthcare monitoring model will offer substantial cost reduction, part of the health data mix will be produced by consumer-grade imaging devices and it needs to be recognized that these often unregulated devices pose an image quality risk. The proposed utility of healthcare digitization will flourish only if the technology isn’t undermined by inappropriate settings and parameterization allowed through unvalidated operation.
Properly configured, these consumer-grade devices can produce images that are not only useful for documentation but can act as data sets to provide much sought after meaningful use through Clinical Decision Support Systems for Evidence-Based Medicine as well as to provide data to support Comparative Effectiveness Research. Beyond validating these imaging devices in their various medical applications, there is a need to ensure that the critical services they enable will scale to where they can reliably serve a 311+ million member healthcare population. These services will require industrial strength solutions. To fill this need, it should be possible to tap into the imaging applications that have been developed at technology engines such as Google, IBM and Microsoft. Their innovations could be used to help facilitate the creation and dissemination of needed services at a scale that could be sufficient to impact even an edifice as consumptive as American healthcare. One such service might be to create a DICOM-quality clinical image database to support EBM in a form that can be distributed to and used by physicians in their clinics to improve performance while minimally disrupting workflow. Another service that could improve clinic workflow would be to provide each patient with an 3D body model that corresponds to individual physiques and that could be integrated with the patient’s PHR. Whatever the particulars of these two examples or the scores of other utilities under consideration and given the current economic and legislative climate, corporate contributions to the national program of healthcare reform will be required for implementation of innovation not to stagnate.
While the impact of dermatology on the consumer image capture and display device markets is not likely ever to become a significant component, it may be the case that even the imaging device manufacturers can yet be drawn to this standards effort for other reasons. Among these reasons is the need to characterize devices for dermatology to a degree and in a fashion not currently required by the consumer market. This new perspective on images is driven by the belief that digital representations in medicine are useful for more than observation – pixels are more than just points of light. Images provide data for computational photography transformations that result in algorithms that operate beyond the ken of the visual system. Improved methods of detection and correction of distortions in the photographic process or the location of salience in an image have the potential to open up a wider marketplace to new tools and applications originally created for dermatology using either in-camera or post-camera processing. For example, the development of an expanded metadata wrapper could open up applications where multiple images are easily linked together to form more complex data structures, even when using images acquired with consumer-grade equipment. This approach may gain purchase in areas as the adaptation of 3D diagnostic tools to cinema and gaming.
In addition, while the capture and transmission of data in home health monitoring and other telehealth applications has been limited to date to scalar and 1D vector values, applications that include imaging are beginning to appear. The source of this data ranges from webcams to industrial-strength teleconferencing systems. What clinical tasks these images will be used for and what degree of regulation they will incur is still unclear. Home telehealth imaging has the potential to develop into a sizable healthcare market segment (a potential that has the same dependence on consumer equipment as does teledermatology). As the reality of the impending healthcare cost crisis becomes ever more immediate, the utility of these monitoring tools to reduce cost will garner increasing attention and resources. In consequence, it may be possible to attract participation and support from the more technologically adept provider organizations and healthcare innovators such as Kaiser Permanente. Several of these organizations already maintain substantial research programs of their own. Much of this research is motivated because payor-provider networks such as Kaiser are in a position to benefit from replacing the fee-for-service paradigm with preventive care and wellness programs. In these new approaches to healthcare, both self and clinical monitoring play a big part in maintaining patient investment and compliance and wherein imaging is playing an increasing role in providing timely and effective feedback.
The new business model for developing dermatologic imaging standards centers on obtaining support from the nontraditional sources described above, first to create and sustain a program of research that characterizes and advances the use of consumer-grade cameras and displays in medical applications and then to organize the research results into a dermatologic imaging standard that enables the use of these consumer devices in a manner that provides accurate and consistent results in the clinic.
The initial focus is to create a
laboratory dedicated to the characterization of consumer-grade cameras and displays
for the use in dermatologic diagnostic imaging. This line of investigation has
already begun in an existing facility, the Skin Appearance Laboratory in the dermatology
department at the
A major goal in the standards project is to define the complete Diagnostic Imaging Chain for Dermatology spanning illumination, tissue optics, BRDF, image capture, image format, image compression, display, viewing environment, observer sensitivity, target salience, and diagnostic decision. The tests and experiments that result from this effort will be focused on supporting the development of a dermatologic imaging standard that covers a set of basic visualization, diagnostic and workflow utilities. Working hand-in-hand with the development of a standard, the new research can be used to support the definition of improved tools and practice. Later, with a standard in place, work in the laboratory would shift to supporting the adoption of the new imaging devices that appear daily from a dynamic consumer market. The low-cost devices would be adapted to meet current and future medical needs through the validation of new methods whose utility would be tested in the clinic. One of the significant benefits of a laboratory situated in a medical school at a university is the access to both clinical and general subject populations.
The current momentum for healthcare reform and the digitization of medicine provides an environment of common interest that spans clinical, scientific, commercial and political factors. More than most anything else, this technological Zeitgeist will bring together potential allies and will elicit subscription and support for ideas where none was forthcoming a decade ago. A list of potential contributors is described in the next section.
Working Group 19 - The prior efforts that successfully created DICOM standards for a number of specialties have faced a somewhat different environment than the one that dermatology currently faces. Advances in dermatologic imaging have foundered because of a lack of perceived need. Added to the lack of motivation is that dermatologists historically have not required expensive diagnostic equipment to form a diagnosis. This results in the absence of the synergies which in other specialties bring together vendors and clinicians. The current lack of clinical technological demand extends even to a near absence of the validated diagnostic use of patient images inserted into the medical record. The major two exceptions are those sites that provide teledermatology services and archive the transmitted images and those sites that store digitized histological images with the patient record.
The use of image processing on clinical data to reduce the morbidity, expense and delays associated with biopsies is commonly no more than an occasional academic exercise. Even so, the straightforward application of consumer-grade cameras and displays designed to produce aesthetically pleasing images has gained uncritical clinical acceptance. Unfortunately, the bulk of the use of these imaging devices in ambulatory clinics results from unvalidated clinical activities. The lack of research on the effect these devices have on specific diagnostic detail can be traced back to the lack of negative findings in outcome studies that look at the dermatologic diagnostic process through the unfocused lens of the wide diversity of medical skin conditions.
If the utility to the clinical workflow of even the basic image processing applications were validated, it would open the door for further research in the development of more advanced digital imaging applications, applications that at the very least would provide due diligence quality assurance measures for the sufficiency of the images captured in the clinic and displayed in the EHRs. Given the combined absence of both vendor champions and any significant native clinical demand, the fabrication of the required tools will need to be created and deployed in an incremental fashion. Fortunately however, the unsustainable increases in the cost of healthcare has led to the institution of external incentives and requirements and, as a result, clinician acceptance isn’t the only current driver for the application of technology to medicine.
As healthcare reform progresses and the adoption of EHRs get integrated into the clinical workflow of more practices and the transition gets beyond the nuts and bolts of basic implementation and connectivity, there will be an increasing opportunity to address more of the potentially transformative issues. These include providing meaningful use by fulfilling the requirements of the health care reform act by incorporating EBM and CE mechanisms as well as quality assurance applications. While these more complex functions will surely take even more time to get right, it is likely that the benefits that emerge from the integrated checks and balances of ancillary utilities that have been shown to drastically reduce morbidity and mortality due to transcription and prescription errors will provide a cushion of acceptance for the introduction of technology in the clinic. There is nothing in nature as powerful as a well-formed feedback loop and the one formed by EBM and CE has the potential to be truly transformative.
In contrast to struggling with fundamental changes in healthcare, it is expected that the analysis of the diagnostic imaging chain will have less earthshaking but more readily implementable insights on improving image quality and diagnostic performance. These findings can be integrated in the dermatology IUGUI in a way that will assist a wide range of quality, diagnostic and workflow functions. Earlier work on the DSC model of teledermatology has achieved a number of successes – technically, clinically and financially. The dermatology IUGUI can form a testbed that will allow new ideas to be examined (measuring not only focus and motion blur and color management but also body mapping trials and medical image quality photography labels) and improved through experience in the clinic. The early delivery of a range of incremental successes in performance should even convert a good portion of the ‘I’ll retire first’ contingent.
Given the change in the business model for standards creation, how does this effect the composition of the skills required to draft a dermatologic imaging standard? At the very least, the added requirement to not only parse and vet the validation research but to also design and execute the studies themselves will necessitate a change in the composition of the membership. It will require technical competence to conduct the device research and also input from the NIH and FDA to provide guidance on clinical best practice and regulatory issues. Traditionally, much of the groundwork of vetting new ideas has been accomplished through interactions between the device manufacturers and the FDA prior to any standards work. This has not been the case for consumer-grade imaging devices. Although there has been recent activity on the use of smartphones for the use of monitoring patients in cardiology and on performing diagnostic reading of some forms of radiological images, there is much activity based on consumer devices that continues to function under a policy of benign neglect.
There also will be a need for DICOM specialists to cement it all into a functioning communications package. Finally, interactions will be needed with Working Groups within DICOM that have overlapping interests as well as with other standards organizations serving the larger healthcare world.
Conclusions - There is a reason why Working Group 19 has made no progress for over a decade in the middle of a digital revolution. Dermatology is missing key incentives and organizational structures that must be put in place before that specialty will move to integrate digital imaging with diagnosis and workflow. The problems impeding the creation of an imaging standard for dermatology can be summed up in seven observations:
1. Although dermatologists capture digital images, they do not commonly depend on them for diagnosis.
2. Since adequate dermatological diagnoses result from face-to-face physical exams, the status quo is viable (but not close to optimal).
3. Therefore, there is no concern about using COTS cameras and displays since the
images are just for documentation and teaching.
4. Use of inexpensive COTS equipment is reinforced because it is all that can be supported by the low reimbursement level for most dermatologic visits.
5. The low level of capital equipment expenditure doesn’t attract sufficient vendor investment.
6. Unfortunately, the lack of vendor investment suppresses the development, validation and general clinical acceptance of applications for the use of digital images in dermatologic diagnosis and visualization.
7. The lack of digital development and validation in dermatology hasn’t caused clinical concern because ... (see 1).
The creation of a dermatologic imaging standard will help break the cycle currently preserved by satisfaction with the status quo and thereby promote the advantages of digital imaging for improved dermatologic diagnostic performance and support a more efficient and economical clinical workflow. To accomplish this goal, a business model must be found that provides the necessary validation and fosters the creation of appropriate dermatologic imaging research. Attempts should be made to obtain support from vendors, not in the traditional mold of depending on those who sell capital equipment that is part of the diagnostic process, but from those who provide services that are advantaged by having all imaging specialties integrated into the same digital healthcare infrastructure.
In addition, the pertinent governmental agencies and specialty societies (e.g., the NIH, FDA, ONC, AAD, and ASDS) must be brought into the standards process early on to provide guidance on best practice, especially for applications and procedures that break new ground. This recruitment will not be easy. In a survey of several thousand physicians, dermatology was the only specialty where not a single practitioner felt the Health Care Reform Act will result in positive outcomes. More enthusiasm will likely be found in recruiting involvement from governmental organizations (e.g., the FDA, ONC and NIH) especially given the recent increase of their support to nontraditional intramural and extramural partnerships.
Nonetheless, many issues remain to be resolved. One issue in particular that needs to be addressed in dermatology is the inclusion in the patient EHR of digital images captured during the course of a face-to-face physical exam. In the descriptions above, these dermatologic EHR images (excepting dermatopathology and teledermatology) have been ascribed the functions of documentation and teaching. The fact that these images are not a principal source of diagnostic information does not mean that there are no image quality requirements to be imposed. When used for documentation, what is being documented? What imaging parameters should have been applied for the relevant clinical details in the images to be salient to any physician opening the record at a later date? Similarly, do the teaching images sufficiently capture all of the diagnostic detail present in the physical lesion? Is there a danger of teaching with exemplars that visibly present only a partial or distorted representation of a lesion or rash?
On the other hand, if no clinically valid reason can be put forth as to why an image should be inserted into an EHR other than the low cost of capture and a vague sense of utility, why incur the expense, or the liability? There is a potential downside to including undocumented images that may only partially present the appearance of a lesion. There is a risk that images will be used in decision processes which they are not capable of supporting, possibly inducing false negatives. Any images included in the medical record should be labeled as to the uses for which they are appropriate, or, at the very least, their metadata should contain sufficient capture and compression parameters to make this determination should the need arise. For both quality assurance and for data security reasons, the EHR should not be a repository for undocumented ‘documentation’ images, nor should it be a teaching image database. This is especially true when the EHR is viewed not only as medical data but also in its role as a legal document. It should be noted that the use of consumer-grade imaging by clinicians is likely just a harbinger of greater problems that will result from home telehealth monitoring and patient-centered care. As the initial hassles of connectivity between clinical EHRs and patient PHRs cease to be an issue, they will be replaced by concerns over the role patient-supplied data (including images) will play in requiring response from the clinician.
There are an additional number of issues that must be resolved before digital dermatology can responsibly move into the clinic. In particular, we should determine whether diagnostic performance would improve if better imaging and analysis systems were available to the dermatologist. In addition, we should investigate whether the improvement in diagnostic accuracy would lead to savings at least equal to the cost of using these systems and maintaining the associated hardware. Or, alternatively, is it the case that dermatologic costs are so low that when viewed in the context of the healthcare budget as a whole, that the status quo is both adequate and appropriate, and the required resources should be spent elsewhere? In my mind, this is still an open question.
This is not to argue that the clinical door should be closed on consumer-based visible light imaging for dermatology. It is to suggest that the advantage of this new technology, the ‘killer app’, may well be found in innovations of care delivery. It may well be the case that some modes of healthcare delivery will be better positioned to take advantage of the new technology than others. In particular, the diagnostic advantage provided by currently available digital analytical tools may not offer a large benefit to an experienced specialist in a face-to-face exam. Consider as an alternative, the advantage these imaging utilities provide in expanding services and reducing costs by supporting the augmentation of scarce specialty services by midlevel staff. Such an expansion in the supply of caregivers could be enabled by the supervisory feedback these digital capabilities afford. Advantages are thereby obtained by increasing the availability of needed clinical services while maintaining quality of care at reduced cost. Additional advantage may be accrued by recruiting PCPs to act as gatekeepers for specialty services and to frequently provide the initial round of care and thus free up scarce specialty services for those cases where they are required. Applying innovation of this sort to the organization of healthcare is only beginning.
Beyond resolving the business case for digital clinical workflow, there is a whole host of technical characterization issues associated with fleshing out the device-related portions of the imaging chain. From my experience, these issues, while requiring a significant amount of analysis, are eminently doable and, in many cases, have similarly been done in the process of creating models of other complex imaging system transfer functions. What is less clear to me is the issue of assessment – assessing clinical performance, assessing image quality, and assessing diagnostic decisions. Images, however, contribute more to healthcare than evidence for diagnosis. They provide objective, quantitative evidence for the efficacy of different treatments and will be a significant contributor to CE measures. They also will provide a source of data for quality assurance. Implementing these issues will involve developing new specifications for meaningful use and this will require breaking new ground.
Early on, much of the performance data for dermatologic digital imaging was anecdotal. As more studies were run, problems remained with performance metrics even when the patient populations were larger. The problems stem from these studies not addressing the effect that the large spectrum of dermatologic diseases has on the validity of performance measures. There is a wide variety in the cases that come through the clinic door – some are acute, some chronic; some are cosmetic, some life-threatening. This diversity reflects the long-tailed disease prevalence distribution of the presented cases. There has been no clear consensus on how to determine what the expected endpoint is given the heterogeneity of the cases, or even what constitutes a successful diagnosis. Should an overlap in the differentials be considered sufficient or does an accurate measure of performance require an explicit single match? Sometimes it doesn’t matter to the course of treatment, sometimes it does; some treatments have broad effects, some are very specific. Weighted equally, the number of cases that do not require exceptional diagnostic performance wash out those that do, but it is the latter group of patients that is generally at greater risk. Curing a case of acne is not the same as curing a case of melanoma – not to the individual patients, and not to the proper evaluation of the healthcare system.
The issue of obtaining a fair measure of healthcare performance goes well beyond establishing what constitutes a diagnosis for a given disease. Absent a validated theory for each disease, the efficacy of drugs, procedures, devices and other therapeutic factors has largely been based on outcome studies. Outcome studies are susceptible to subjective judgments for the rules defining the quality and adequacy of the data to be included. Concerns have long been raised over the design and execution of performance-based studies when the individuals involved have a philosophical predisposition for the issues or a clear financial stake in the results. This is especially a concern when research funding comes from industry. This trend shows no signs of abating. Concerns have also been raised over the development of medical guidelines and for some of the same reasons. Guideline committees sanctioned by professional societies made up of their members who have a direct economic interest in the specifications of those guidelines cannot be considered objective parties. This is especially troubling when all the seats at the table are filled by members of the same constituency of stakeholders.
As an alternative to these outcome-based studies and guidelines, models are often developed using first principle analyses to predict the range of potential outcomes. These analyses depend on computations that use basic physical and biological properties of system components that are usually very well defined. The resulting simulations act as checks on the claims made by performance studies, and vice versa. Neither perspective is free of the risk of manipulation of the data by an unbalanced structuring of the studies. It should be acknowledged that the presence of such influences is not necessarily an indication of malicious intent. Individuals will always have personal points of view that influence their choices. In this regard, the two approaches to research offer a useful counterpoint to one another. If the observed performance cannot be matched to realistic combinations of the constituent components or if the simulations predict performance well beyond or below anything ever observed, caution should be used in deploying the results in the clinic.
The occurrence of such discrepancies is not an idle concern. Consider the recent defense of the benefits of EHRs by the new National Coordinator of Health Information Technology, Farzad Mostashari. He has attributed the recent abundance of negative findings to insufficient study size and the use of flawed and biased outcome study designs. Especially in times of paradigm change, conventions are in flux and these study design miscues are not always recognized and weeded out in the editorial process. Only recently have studies emerged of sufficient size and appropriate design and they tend to support EHRs strongly. Positive EHR results notwithstanding, sources of evidence and analysis beyond outcome studies should be considered before embracing desired results.
Consider also the recent rapid transition from where properly illuminated reading rooms and high-end medical monitors were accepted as the gold standard for primary reading of radiological images for over two decades. Consider now where the near universal acceptance of anywhere viewing of consumer-grade smartphones is approved for image-based diagnosis. This adoption is not just for secondary viewing for patient positioning or for scheduling studies. This is for FDA-approved primary diagnostic decision making for CT, MRI and PET scans on a 0.6 megapixel, 4.5 by 2.3 inch handheld display. The question is not simply whether Apple’s Retina Display can adequately represent diagnostic detail. It is also about how two decades – scores and scores – of first-rate medical display performance studies were summarily dismissed in the rush to promote the new mHealth devices.
This is not the only example of medical imaging diagnostic inroads made by consumer devices. Marked increases in growth of teleradiology over the last decade is the result of the ability to electronically transmit digital radiological images to distant sites for reading. This capability has enabled better coverage in rural areas but also has raised several issues – including the credentialing and privileging of remote consultants across state lines. Hopefully, state legislatures will come to recognize that preserving the state’s prerogative to control medical licensure is not as important as gaining access to nonuniformly distributed radiological skills that can save their constituents’ lives.
There is another issue, however, that has not received as much public scrutiny. Particularly in rural areas where coverage is low, the practice of sending images to radiologists off-site for a timely reading has also increased. This results in readings where the quality of both the viewing environment and of the electronic display being used is not likely to match that of a radiology reading room. While the use of most any resources in an emergency is justified, this practice has no history of due diligence by requiring subsequent corroboration of the off-site results with sanctioned equipment in a timely fashion.
Assessment of image quality and imaging device performance is especially important for telemedicine. As Program Director for VISN 2 Telemedicine at the Department of Veterans Affairs, I worked on the development of teledermatology clinics. [58] As part of this project, we considered a metric that was based on lesion cases (omitting rashes). Rashes tend to be chronic conditions, often with no expectation of a clear endpoint. The selection of a lesion-centric metric may not provide a measure covering the entirety of cases that pass through the exam room door but it does have the advantage of providing a clearer measure of the time savings the efficiencies of our teledermatology implementation produced. Proper interpretation of existing studies, and the proper design of future ones, will require consensus to be achieved on this issue. Defining the judgments of what visual evidence is required to form dermatologic diagnoses and of what constitutes proper operation of the consumer-grade imaging devices and procedures used to obtain those diagnoses is central to the work proposed here. It is also an area that would be appropriate for the NIH and professional societies to address to determine the degree to which new findings in skin research and new imaging modalities can be combined to improve dermatologic diagnosis.
The core capability behind all of the utilities and services depends on the development of a detailed and comprehensive model for the assessment of the image-based diagnostic decision process. Central to this development is a visual system-based model that combines luminance contrast and chromaticity into a unified detection and discrimination model (e.g., [59]). To help in the understanding of the diagnostic process, this model can be applied to the analysis of potential masking effects induced by the tissue surrounding the diagnostic morphology, thereby altering performance. The obtained vision metrics can then be extended to provide the responses that form the basis of components in the diagnostic imaging chain, including models of visual search and other higher-level diagnostic decision processes. [60-62] In measuring the performance of the diagnostic imaging chain, it is important to apply a vision metric that embodies the same pattern of sensitivities present in the clinical observer. Over the last quarter century, there has been no end of computer vision and machine learning algorithms brought to bear on assessing visual performance. In retrospect, no matter how efficient their radiometric performance is deemed to be, none of them cleave to the variations of human performance anywhere near as well as those metrics based on visual physiological and psychophysical data. With the imaging chain tested and validated with a vision-based metric, the results will be integrated into the various analytic tools and measures of the IUGUI.
With these diagnostic imaging chain components defined, it will be possible to begin a first principle assessment of image quality requirements that cover the variety of spatial and chromatic morphology present in images of benign and diseased skin tissue. In particular, it is important to know to what degree the contribution of the diagnostic cues is degraded by camera motion, defocus or any of the manipulations performed on the images by the cameras and displays by in-device processing. This issue needs to be addressed principally to protect patients from diagnostic decisions that are false negatives and that are the result of the use of inappropriate techniques or undocumented imaging practices that are now commonly in place.
Analogous to the implementation of HDTV, the benefits of switching to a digital format for medical imaging diagnosis will come downstream for dermatology. With HDTV, the crucial factor isn't the effects of a small increase (4X) in pixels; it is the ability of the digital format to support interactive TV, such as clicking on an item while watching a show and by doing so purchase an equivalent item. Similarly, the use of digital images in dermatologic diagnosis will ultimately support analytical and visualization utilities that, in turn, will facilitate even better diagnostic performance.
Not surprisingly, the digital revolution that has produced substantial advantages for our culture and for our economy also has had, and likely will continue to have, a profound impact on the delivery of healthcare. These advances are due in particular to the greater utility of what has become an increasingly critical form of medical data, digital imaging. This utility will be multiplied by better accessibility to that diagnostic information by both the clinician and the patient through the use of EHRs and PHRs. These digital advances in imaging will offer improvements in care through better systems analysis, clinical collaboration and coordination, and will facilitate the creation of more sophisticated diagnostic aids. For this to work to advantage in today’s healthcare enterprise, all specialties must be included. For dermatology, much of this promise may well yet come to fruition; however, there is considerable work that needs to be done here and now to provide for a solid foundation. In particular, what is needed to responsibly bring digital diagnosis into the dermatology clinic is a fully configured Diagnostic Imaging Chain Model and the means to validate it.
In conclusion, it should be remembered that beyond all other considerations, the fundamental goal of a medical standard is not simply to ensure the nuts and bolts of interoperability or to promote efficiency in care delivery. The fundamental goal behind all decisions must be the protection of the patient. This assertion is of importance here because when it comes to the consequences of technology and medical imaging, everyone defers to DICOM.
Acknowledgments
I very much would like to thank
the following people for their advice and comments: Alice Pentland, Christopher
Brown, Joseph Kvedar, Lowell Goldsmith, Allan Halpern, Nikiforos Kollias,
Anthony Gaspari, Peter Lio, Kenneth Warrick, Elizabeth Krupinski, Peter Kuzmak,
William Stoecker, Matthew Fleming,
References
[1] David Bandon, et al. (2005) Enterprise-wide PACS: Beyond radiology, an architecture to manage all medical images. Academic Radiology, 12:1000-1009.
[2]
Bjorn Bergh (2006)
[3]
James E. Silberzweig, et al. (2007) The use of a picture archiving and communication
system to catalogue visible-light photographic images. Journal
of Vascular
and Interventional
Radiology, 18:577-579.
[4] James W. Woods, et al. (2006) Using UMLS metathesaurus concepts to describe medical images: Dermatology vocabulary. Computers in Biology and Medicine, 36:89-100.
[5] Klaus Wolff, Lowell Goldsmith, Stephen I. Katz, Barbara A. Gilchrest, Amy Paller and David J. Leffell (2007) Fitzpatrick’s Dermatology in General Medicine, 7th Edition, McGraw-Hill Professional.
[5½] Hany Farid (2008)
Digital image ballistics from JPEG quantization: A follow-up study. Computer
Science,
[6] James R. Janesick (2001) Scientific Charge-Coupled Devices, SPIE Press.
[7]
Junichi Nakamura (Ed.) (2006) Image Sensors and Signal Processing for Digital
Still Cameras,
[8] Orly Yadid-Pecht and Ralph Etienne-Cummings (Eds.) (2004) CMOS Imagers: From Phototransduction to Image Processing, Kluwer Academic Publishing.
[9] Bruce Fraser and Jeff Schewe (2008) Camera Raw with Adobe Photoshop CS3, Peachpit Press.
[10] Alexander Hornberg (Ed.) (2006) Handbook of Machine Vision, Wiley-VCH.
[11] Leinweber, et al. (2006) Teledermatopathology: A controlled study about diagnostic validity and technical requirements for digital transmission. American Journal of Dermatopathology, 28:413-416.
[12] Domingo DeAgustin, et al. (2008) Technological bases (sic) for teledermatopathology: State of the art. Seminars in Cutaneous Medicine and Surgery, 27:25-31.
[13] Cesare Massone, et al. (2008) Teledermatology: An update. Seminars in Cutaneous Medicine and Surgery, 27:101-105.
[14] Elizabeth Krupinski, et al. (2007) American Telemedicine Association’s Guideline for Teledermatology, www.atmeda.org/ICOT/Standards/Telederm_guidelines_v10final.pdf, (accessed 16 October 2008).
[15]
[16] Stefan Winkler (2005) Digital Video Quality, John Wiley & Sons, Ltd.
[17] Alexander Roesch, et al. (2006) Dermoscopy of “dysplastic nevi”: A beacon in diagnostic darkness. European Journal of Dermatology, 16(5):479-493.
[18]
Josep Malvehy, et al. (2007) Dermoscopy report: proposal for standardization. Results
of a consensus meeting of the International Dermoscopy Society. Journal of the
[19] Allan C. Halpern and Jocelyn A. Lieb (2007) Early melanoma diagnosis: A success story that leaves room for improvement. Current Opinion in Oncology, 19:109-115.
[20] Malene E. Vestergaard and Scott W. Menzies (2008) Automated diagnostic instruments for cutaneous melanoma. Seminars in Cutaneous Medicine and Surgery, 27:32-36.
[21] Andreas Blum, Iris Zalaudek and Guiseppe Argenziano (2008) Digital image analysis for diagnosis of skin tumors. Seminars in Cutaneous Medicine and Surgery, 27:11-15.
[22] Josep Malvehy and Susana Puig (2002) Follow-up of melanocytic skin lesions with digital total-body photography and digital dermoscopy: A two-step method. Clinics in Dermatology, 20:297-304.
[23] Neal E. Feit, Stephen W. Dusza and Ashfaq A. Marghoob (2004) Melanomas detected with the aid of total cutaneous photography. British Journal of Dermatology, 150:706-714.
[24]
Adriana Olmos and
[25] Bertha B. Lin and R. Stan Taylor (2001) Digital Photography for mapping Mohs micrographic surgery sections. Dermatologic Surgery, 27:411-414.
[26] Howard D. Krein, Steven S. Greenbaum and David Reiter (2005) Color-specific enhancement of digital photographs for identification of the extent of cutaneous malignancy. Archives of Facial Plastic Surgery, 7:135-137.
[27] Steven L. Jacques, Jessica C. Ramella-Roman and Ken Lee (2002) Imaging skin pathology with polarized light. Journal of Biomedical Optics, 7(3):329-340.
[28] Heesung Kang, Byungjo Jung and J. Stuart Nelson (2007) Polarization color imaging system for on-line quantitative evaluation of facial skin lesions. Dermatologic Surgery, 33:1-7.
[29] Sergio G. Coelho, et al. (2006) Quantification of UV-induced erythema and pigmentation using computer-assisted digital Image evaluation. Photochemistry and Photobiology, 82:651-655.
[30] Thilo Gambichler, et al. (2006) A comparative pilot study on ultraviolet-induced skin changes assessed by noninvasive imaging techniques in vivo. Photochemistry and Photobiology, 82:1103-1107.
[31] Wang Lingua and Graham Leedham (2006) Near- and far-infrared imaging for vein pattern biometrics. Proceedings of the IEEE International Conference on Video and Signal Based Surveillance.
[32] Yuta Miyamae, et al. (2008) A noninvasive method for assessing interior skin damage caused by chronological aging and photoaging based on near-infrared diffuse reflection spectroscopy. Applied Spectroscopy, 62:677-681.
[33] Kishwer S. Nehal, Dan Gareau and Milind Rajadhyaksha (2008) Skin imaging with reflectance confocal microscopy. Seminars in Cutaneous Medicine and Surgery, 27:37-43.
[34] Salvador Gonzalez and Yolanda Gilaberte-Calzada (2008) In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. International Journal of Cosmetic Science, 30:1-17.
[35]
Thilo Gambichler, et al. (2007) Characterization of benign and malignant melanocytic
skin lesions using optical coherence tomography Vivo. Journal of the
[36] Milos Todorovic, et al. (2008) In vivo burn imaging using Mueller optical coherence tomography. Optics Express, 16(14):10279-10284.
[37]
Benjamin Albouy, Yves Lucas
and Sylvie Treuillet (2007) 3D modeling from uncalibrated color images for a complete
wound assessment. Proceedings of the 29th
Annual International Conference of the IEEE EMBS Cite Internationale,
[38] Chulhyun Ahn and Richard Salcido (2008) Advances in wound photography methods. Advances in Skin & Wound Care: The Journal for Prevention and Healing, 21(2):85-93.
[39] Monika-Hildegard Schmid-Wendtner and Dorothee Dill-Muller (2008) Ultrasound technology in dermatology. Seminars in Cutaneous Medicine and Surgery, 27:44-51.
[40] Francisco Bobadilla, et al. (2008) Pre-surgical high resolution ultrasound of facial basal cell carcinoma: Correlation with histology. Cancer Imaging: 8(1):163-172.
[41] Ya-Hui Shiao, et al. (2007) Quality of compressed medical images. Journal of Digital Images, 20(2):149-159.
[42] Fabrizio Guarneri, Mario Vaccaro and Claudio Guarneri (2008) Digital image compression in dermatology: Format comparison. Telemedicine and e-Health, 14(7):666-670.
[43] Wendy L. Parker, et al. (2007) Objective interpretation of surgical outcomes: Is there a need for standardizing digital images in the plastic surgery literature? Plastic and Reconstructive Surgery, 120(5):1419-1423.
[44] Stephanie Ogden and T. W. Griffiths (2008) A review of minimally invasive cosmetic procedures. British Journal of Dermatology, 159(5):1036-1050.
[45] Charles E. Gessert and Joel T. M. Bamford (2003) Measuring the severity of rosacea: A review. International Journal of Dermatology, 42:444-448.
[46] Susan Y. Quan, et al. (2007) Digital imaging of wounds: Are measurements reproducible among observers? International Journal of Lower Extremity Wounds, 6(4):245-248.
[47] Subok Park, Aldo Badano, Brandon D. Gallas and Kyle J. Myers (2009) Incorporating human contrast sensitivity in model observers for detection tasks. IEEE Transactions on Medical Imaging 28(3):339-347.
[48] Hossein Ragheb and Edwin R. Hancock (2008) A light scattering model for layered dielectrics with rough surface boundaries. International Journal of Computer Vision, 79:179-207.
[49] Alice N. Averbukh, David S. Channin and Michael J. Flynn (2003) Assessment of a novel, high-resolution, color, AMLCD for diagnostic medical image display: luminance performance and DICOM calibration. Journal of Digital Imaging, 16(3):270-279.
[50] Hans Roehrig, et al. (2006) In-field evaluation of the modulation transfer function and the signal-to-noise ratio of electronic-display devices. Journal of the SID, 14(10):847-860.
[51] Elizabeth A. Krupinski, Jeffrey Lubin, Hans Roehrig, Jeffrey Johnson and John Nafziger (2006) Using a human visual system model to optimize soft-copy mammography display: Influence of veiling glare. Academic Radiology 13:289-295.
[52] Anindita Saha, Hong Liang, Rebecca Vogel and Aldo Badano (2008) Assessment of mobile technologies for displaying medical images. Journal of Display Technology 4(4):415-423.
[53]
Brian C. Madden and Ulf M. Cahn von Seelen (1995) PennEyes – A binocular active vision system. Technical Report MS-CIS-95-37/GRASP LAB 396, December 1995, Computer and
Information Science,
[54] Jarmo Ilonen, et al. (2008) Image feature localization by multiple hypothesis testing of Gabor features. IEEE Transactions on Image Processing, 17(3):311-325.
[55] Tanja C. W. Nijboer, et al. (2008) Recognising the forest, but not the trees: An effect of colour on scene perception and recognition. Consciousness and Cognition, 17:741-752.
[56] Ning Situ, Xiaojing Yuan, Ji Chen and
George Zouridakis (2008) Malignant melanoma detection by bag-of-features
classification. 30th Annual
International IEEE EMBS Conference,
[57] Sophie Girardi, et al. (2006) Superiority of a cognitive education with photographs over ABCD criteria in the education of the general population to the early detection of melanoma: A randomized Study. International Journal of Cancer, 118:2276-2280.
[58]
Brian C. Madden (2009) The Use of non-specialty staff for teledermatology in
the Veterans Health Administration. Technical Report, Skin Appearance
Laboratory, Department of Dermatology,
[59] Sophie M. Wuerger, Andrew B. Watson and Albert Ahumada (2002) Towards a spatio-chromatic standard observer for detection. Human Vision and Electronic Imaging VII, Proceedings of SPIE, 4662:159-172.
[60] Joost van de Weijer, Theo Gevers and Andrew D. Bagdanov (2006) Boosting color saliency in image feature detection. IEEE Transactions on Pattern Analysis and Machine Intelligence, 28(1):150-156.
[61] Lior Elazary (2008) Interesting objects are visually salient. Journal of Vision, 8(3):1-15.
[62] Wolfgang Einhauser, Ueli Rutishauser and Christof Koch (2008) Task-demands can immediately reverse the effects of sensory-driven saliency in complex visual stimuli. Journal of Vision 8(2):1-19.